NNadir
NNadir's JournalNobel laureates and science groups demand NIH review decision to kill coronavirus grant
This is in the news section of the current issue of Science. I believe it may be open sourced.
The text is here: Nobel laureates and science groups demand NIH review decision to kill coronavirus grant
An excerpt:
Thirty-one scientific societies have also written to Collins, calling on NIH "to be transparent about their decision-making process on this matter... The action taken by the NIH must be immediately reconsidered."
On 24 April, NIH informed the nonprofit EcoHealth Alliance, led by wildlife disease specialist Peter Daszak, that it was ending a grant, first awarded in 2014 and renewed in 2019 because it no longer aligned with the agency’s priorities. The move came after conservative U.S. politicians and media suggested—without evidence—that the coronavirus causing the pandemic escaped from a laboratory in Wuhan, China, that employs a Chinese virologist who had received funding from the grant. The termination also came 1 week after President Donald Trump, when asked about the project at a press conference, said: “We will end that grant very quickly.”
In their letter, the Nobel laureates say they “are gravely concerned” about that decision. “We believe that this action sets a dangerous precedent by interfering in the conduct of science and jeopardizes public trust in the process of awarding federal funds for research. … Now is precisely the time when we need to support this kind of research if we aim to control the pandemic and prevent subsequent ones.”
Never before in the history of our country, has there been a "President" bearing so much contempt for science.
If this situation is allowed to prevail, we are in for a very dark age.
History will not forgive us, nor should it.
Jeeze, I hope my son doesn't ever tell me he's going to graduate school at Stanford.
He hasn't expressed any interest in going there, thankfully, even though one of the most cited Materials Scientists in the world, Yi Cui is a professor there.
Dr. Cui is a very impressive fellow, by the way, having established the conditions under which N95 masks can be sterilized and reused. (He's also a protege of Obama's first Energy Secretary, Nobel Laureate Steven Chu.)
Nevertheless, no reflection on Dr. Cui, Stanford seems to be losing scientific credibility.
Of course, this is the university where the right wing Hoover Institution resides, and of course, people associated with Hoover with no training in epidemiology have been very Trump like in their predictions of how bad the virus would be.
Apparently, for financial incentives, funded by the CEO of Jet Blue, they've been pushing the idea that Covid-19 isn't all that risky:
How Stanford Lost Its Soul
Subtitle: A distinguished university known for its embrace of corporate funding has come down with a bad case of Covid-19 contrarianism.
Stanford is also the home of Mark Z. Jacobson, whose approach to science includes filing a lawsuit against prominent scientific journal for um, publishing science he didn't like.
My son hasn't expressed any interest in Stanford and in any case is up for a scholar award covering his graduate program at his current institution, but if he did...
...under the right circumstances his father can go ballistic.
Limited Genetic Diversity Preceded Extinction of the Tasmanian Tiger.
I was motivated to look into the fate of this animal by a post here in the science section, this one: Newly Released Rare Video Is The Last Known Footage of a Tasmanian Tiger (Thank you Judi Lynn!)
Apparently the animal underwent two extinction events, the first on the Australian mainland owing to the arrival of the dingo subspecies of the dog, about 5000 years ago, the second extinction - which was a deliberate extinction on behalf of Tasmanian sheep herders - in the early 20th century.
One thing that is often overlooked in near extinction of animals like Cheetahs (which apparently had an earlier near extinction event before the current one), the California Condor, and other animals, is that their surviving populations are weakened by a loss of genetic diversity.
The low genetic diversity of the Tasmanian tiger, a marsupial dog like animal, that survived on Tasmania until the 20th century, was established in a paper published in the open sourced journal PLOS One. The paper is here: Limited Genetic Diversity Preceded Extinction of the Tasmanian Tiger (Brandon R. Menzies1,2*, Marilyn B. Renfree2, , Thomas Heider3, Frieder Mayer4, Thomas B. Hildebrandt1, Andrew J. Pask3, One April 2012 | Volume 7 | Issue 4 | e35433) The authors made their study by obtaining tissue samples of the animal from museums.
An excerpt:
Once found on mainland Australia, a subpopulation of thylacines became isolated on the island of Tasmania after the flooding of Bass Straight approximately 10–13 thousand years ago, and so they avoided the decline and eventual extinction of the mainland population that coincided with the arrival of the dingo, Canis lupus dingo, 5–6 thousand years ago [2]. Based on limited observations and bounty information, the thylacine appeared to be a solitary ambush predator that preferred open woodland habitat. Its former range included the north-western, central, eastern and south-eastern parts of the island of Tasmania, but not the mountainous south-west (Figure 1b) [3], [4].
Far from being appreciated, European settlers deemed the thylacine a threat to the developing colonial sheep industry and it was aggressively targeted for eradication by the government with a £1 bounty paid for every animal killed [3]. Mothers with pouch young or live specimens could be sold to zoos or museums for even greater remuneration. As a result, the remaining population was rapidly exterminated from Tasmania during the bounty period from 1888 to 1912, over which time 2,184 specimens were presented for reward (Figure 1c) [3]. The last known wild thylacine was killed in 1930 and the last known animal died in the Hobart Zoo in Tasmania on the 7th of September 1936 [4].
Currently the other marsupial that survived in Tasmania after extinction in Australia, the Tasmanian Devil, also lacks in genetic diversity, which may be a reason that the animal is facing extinction as a result of a highly transmissible facial cancer.
PLOS One is a very fine open sourced peer reviewed journal with which one can broaden his or her scientific knowledge by reading original papers for free.
Have a nice day.
Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement
The paper to which I'll refer is this one: Temporary reduction in daily global CO2 emissions during the COVID-19 forced confinement (Corinne Le Quéré et al., Nat. Clim. Chang. (2020) https://doi.org/10.1038/s41558-020-0797-x)
The full article is open sourced, and anyone can read it. The introductory text of course, includes reference to so called "renewable energy, and endorses the idea that it is becoming cheap and plentiful, neither of which is true on any scale other than one involving wishful thinking and selective attention. Without referring to the first causes which may call this claim into question, the article correctly notes that nothing about the massive worldwide "investment" in so called "renewable energy" has arrested the rise in the use of dangerous fossil fuels, nor has it done anything to address climate change.
An excerpt from the introduction:
The emergence of COVID-19 was first identified on 30 December 20198 and declared a global pandemic by the World Health Organization on 11 March 2020. Cases rapidly spread, initially mainly in China during January, but quickly expanding to South Korea, Japan, Europe (mainly Italy, France and Spain) and the United States between late January and mid-February, before reaching global proportions by the time the pandemic was declared9. Increasingly stringent measures were put in place by world governments in an effort, initially, to isolate cases and stop the transmission of the virus, and later to slow down its rate of spread. The measures imposed were ramped up from the isolation of symptomatic individuals to the ban of mass gatherings, mandatory closure of schools and even mandatory home confinement (Table 1 and Fig. 1). The population confinement is leading to drastic changes in energy use, with expected impacts on CO2 emissions.
As I've noted in several posts in this group, the rises in carbon dioxide as measured at the Mauna Loa CO2 observatory do not show very much change over other years; a new record of 418.83 ppm a few weeks back, for that particular record setting week, 2.72 ppm higher than the same week last year.
New Weekly CO2 Concentration Record Set at the Mauna Loa Observatory 416.83 ppm.
It is important to note that the overwhelming majority of the dangerous fossil fuel waste carbon dioxide dumped by humanity has been extracted into the ocean. Even if there were no further emissions, it is unlikely that the concentration would fall tremendously, since these concentrations are subject, to a first approximation, to Henry's Law, a law of chemical physics that indicates that the solubility of a gas in a liquid is directly proportional to the partial pressure of the gas. This means that if the partial pressure of CO2 were reduced, it would be made up by out gassing from the ocean.
This figure from the Nature Climate Change paper cited in this post, refers to the proportion of carbon dioxide represented by countries in one of three Covid related restrictions.
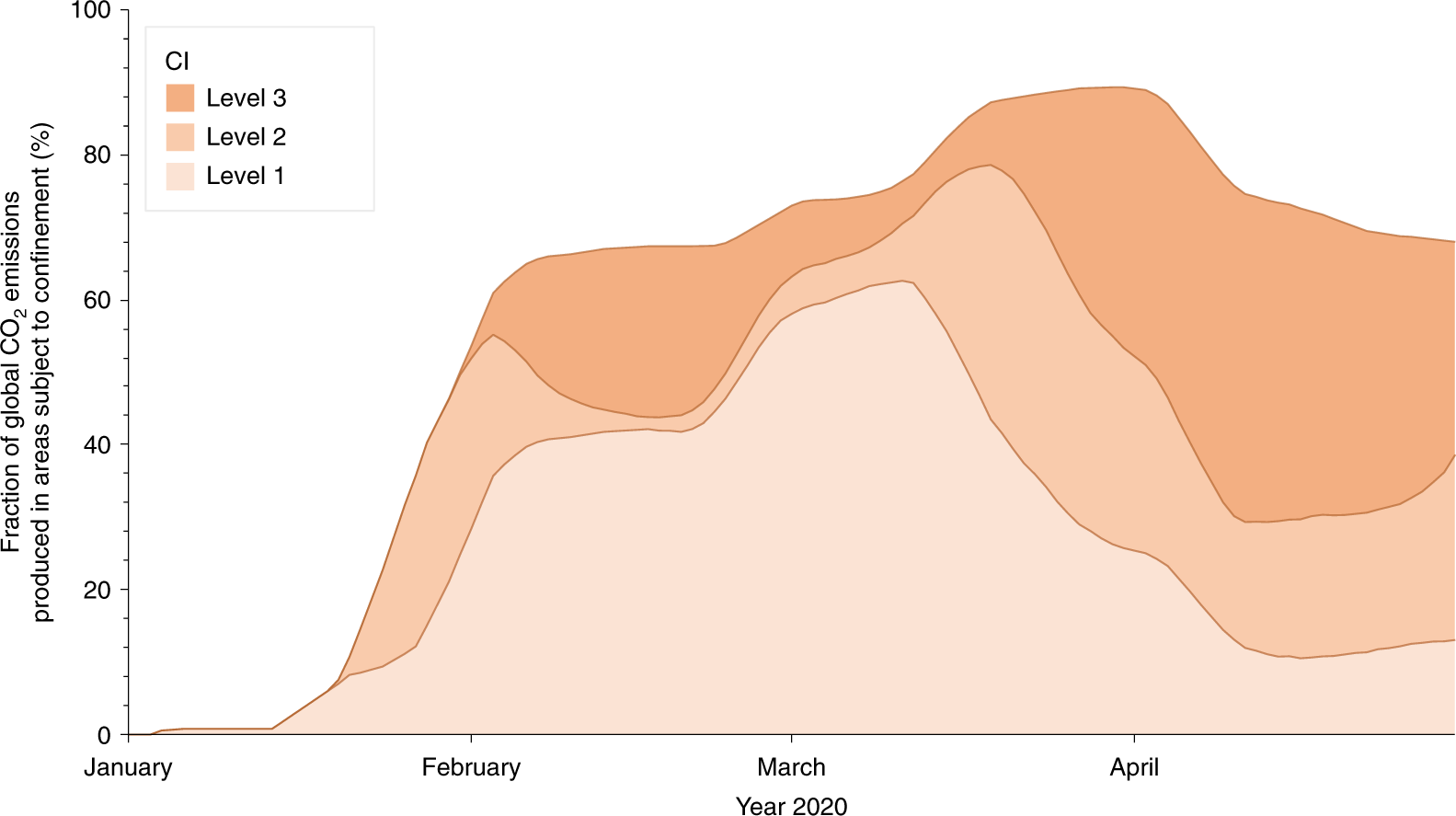
Again, the full paper is available. If interested, you can read it yourself.
It is possible that there will be some measurable effect in the atmosphere. I note that things were looking terrible earlier this year in the weekly data; for the week of March 22, 2020, we actually saw a reading of 4.28 ppm over the same week of the previous year, the 11th highest reading ever recorded. Maybe we can hope, under the circumstances for "not the worst of the worst."
Eventually things will return to "normal," "normal" including the difficult to shake almost theological belief that so called "renewable energy" will displace fossil fuels. It hasn't; it isn't; and it won't.
I trust you're safe and well, and enjoying those things that still can be enjoyed.
The Horror: A NY Times Video of Two College Students Who Took Emergency Morgue Jobs in NYC.
The fine and beautiful two young people took the job of handling the overwhelming flood of dead bodies in NY.
It's disturbing, of course, but very moving.
The video is at this link:
She Spent Her Last Month of College Lifting Bodies in a Morgue
Assholes in the town in which I grew up, harass a reporter covering them.
I definitely know the type, motorhead greasers with poor educations and big stupid mouths. While I knew many fine people when I was growing up, there was a fair share of these types.
Commack, Long Island:
News reporters become subjects of ire at anti-lockdown protests
Oh well, not that they are bright enough to know it, Darwin will win with this selection pressure.
The Preparation of Ultra High Purity Tungsten Free Molybdenum.
The paper I'll discuss in this post is this one: Sustainable Extraction and Complete Separation of Tungsten from Ammonium Molybdate Solution by Primary Amine N1923 (Zhang et al., ACS Sustainable Chem. Eng. 2020, 8, 18, 6914-6923).
In the periodic table the lanthanides, the elements, shown in the graphic below, sequentially add electrons to f orbitals, which in general do not contribute significantly to their bonding.

The directional nature of the f orbitals results in incomplete shielding from the nuclear charge for the outer electrons resulting in what is known as the "lanthanide contraction," a decrease in the atomic radius of the elements and their ions. This has an effect on the chemistry of the elements that emerge sequentially after the lanthanides, halfnium, tantalum, and tungsten since as a result of the contraction, the atomic radius is very close to their cogener elements, respectively, zirconium, niobium and molybdenum. The similarity in size makes the separation of high purity samples of these elements difficult. In fact, the name of tantalum, a "conflict metal" that is an important component of cell phones, derives from the greek myth of Tantalus, because the process of isolating pure tantalum was frustrating. (This difficulty would be expected for the separation of rhenium from technetium, but technetium does not occur naturally except in small traces in uranium ores which generally do not contain rhenium. Therefore this difficulty is not observed in isolating rhenium from its rare ores, although this separation might become important in a future wherein wisdom prevails, not that history suggests that periods of international wisdom are rare.)
Although in my daily ruminations I am far more interested in tungsten than I am in molybdenum, molybdenum is a very important element, and is widely used in alloys and in applications as a refractory in high temperature systems. Pure molybdenum has the sixth highest melting point of the commonly available elements; only tantalum, osmium, rhenium, its cogener tungsten, and carbon have higher melting points. In addition, molybdenum oxides, molybdenates, are important catalysts for many reactions. (The tungstates are also used in many settings.)
(An interesting fact about molybdenum is that it is the heaviest element, except for iodine, that was essential to life before the invention of the Haber process in the early 20th century. Molybdenum coordinating proteins fix nitrogen in the natural world. A few proteins containing tungsten are known is rare species, but they are not nearly as important as molybdenum containing nitrogenases.)
In any case, the authors of this paper describe some of the applications of very high purity molybdenum. From the introduction to the paper:
The nation for which molybdenum is a national strategic resource as discussed in this paper is China, where incidentally, molybdenum is mined; China has the world's largest reserves of this element. (The United States also has significant reserves, albeit, ironically, low in Tungsten.)
The author's review methods by which molybdenum and tungsten are separated when separation is required. Since these methods cannot be metallurgical, they require significant chemistry, and include solvent extraction, selective crystallization, adsorption and ion exchange methods.
The author's here have opted to refine an extraction method, where the extractant is a commercially available reagent known as "primary amine 1923" which is widely utilized in metal extraction chemistry. The name refers to an amino group located on a carbon that features long aliphatic carbon chains on either side. These chains range between 19 and 23 carbons in length, hence, the "1923." By a careful theoretical evaluation of the equilibria obtained in the complex molybdenate/tungstate oxo anion system, they have worked to improve the environmental impact of this separation, in particular by recovery and reuse of primary amine 1923.
The authors write:
The authors, using theoretical and experimental tools propose to govern the extraction with highly accurate and precise control of the pH of the solutions.
The chemistry of the oxoions of molybdenum and tungsten is very complex below pH 8, and highly pH dependent and even more complex when the elements are both present. This is because the oxoions can form polymeric species with themselves and with each other.
Table 2 from the paper details some species found in the solutions:

The (p, q, r) values listed in the table reflect the following equation:

To get a feel for the complexity of this system, consider the equation for the total concentration of Tungsten, [W]T:

The equation for total Molybdenum is similar. Note that the terms with large exponents in these equations appear along side terms for the hydrogen ion concentration, which is obtained from the pH. This expression, and the corresponding expression for total Molybdenum demonstrate the sensitivity of concentrations to pH.
This graphic from the paper shows the pH dependency of this system's components:

The caption:
The next figure shows the species dependence on pH at differing initial concentrations of total tungsten:

The caption:
Next the separations as a function of extractant volumes between the aqueous and organic (primary amine N1932) layers:

The caption:
The following graphic gives a breakdown of W/Mo extraction ratios as a function of pH using different extraction conditions:

The caption:
All of this leads to a flow chart for the separation process:

The caption:
This table shows the conditions and purity of molybdenum under these conditions obtained in these processes:

After much research in the 1950's, in the 1960's scientists and engineers at Los Alamos National Laboratory ran a nuclear reactor, the LAMPRE reactor, using liquid eutectic of plutonium iron metal as a fuel. The metal chosen to contain the plutonium containing the eutectic was tantalum. Tantalum is a conflict metal; it's use is problematic because of conditions of essentially human slavery where its ores occur in the largest amounts. I personally do not feel comfortable encouraging new uses for tantalum. Moreover, it is a fairly rare metal, subject to depletion.
Nevertheless, more than half a century latter, it occurs to me that the idea behind the LAMPRE reactor had much to recommend it. The rising availability of plutonium that has a wide distribution of isotopes, particularly in used MOX fuels, on an industrial scale, makes the idea even more attractive. This would be particularly true if humanity decides on nuclear weapons disarmament, not a likely outcome, since the value of wisdom is declining, not rising, but still an outcome for which we can hope. The use of plutonium with a large distribution of isotopes makes instantaneous denaturation of weapons grade plutonium possible.
The threat of climate change, is of course, worse than the threat of nuclear war, since climate change is observed and nuclear war is not. Climate change is a certainty; nuclear war a possibility. We can, and should, in a time of wisdom, lower the probability of nuclear war by converting weapons grade plutonium into reactor grade plutonium.
All of this relates to tungsten, because during research at Los Almos in the 1950's, it was discovered that tungsten had low solubility in liquid plutonium. The reason that tantalum was chosen over tungsten is that while tungsten has the highest melting point of all known metals, it is very difficult to machine. The machinability of tungsten can be greatly improved by addition of rhenium, but rhenium is a very expensive and very rare element.
However, technetium, a component of used nuclear fuel that is often regarded in what I regard as abysmal ignorance, as so called "nuclear waste" is an excellent substitute for rhenium in almost all applications.
The amount of technetium available in the 1950's was tiny; it is now available on a ton scale.
There are many different ways to contain liquid plutonium I expect, including many types of materials that were unknown in the 1950's or available at only a tiny scale, nanolayered ceramics, metalloceramics such as the MAX phases. It is also conceivable to explore peritectic systems to address the corrosive nature of liquid plutonium.
However machinable alloys of tungsten are an excellent default.
In "breed and burn" nuclear systems containing tungsten as a structural component - systems designed to operate for many decades without refueling - tungsten, which is relatively rare but certainly available in industrial quantities, particularly in mass efficient systems like nuclear reactors, will be slowly converted into the far more valuable metals rhenium, osmium and iridium. This of course, would be a good thing.
Tungsten alloyed with technetium, and placed under neutron irradiation for a fair portion of a century will also contain the metal ruthenium from the transmutation of technetium. Ruthenium is another valuable metal which is also a fission product. However in recovering the rhenium some residual technetium will remain, and therefore, the separation of rhenium and technetium, not observed in nature, may require similar study to that conducted here for molybdenum and tungsten here.
All of this is esoteric I know, and has little to do with politics, but it shows some interesting light that may someday pierce the current darkness in a better world.
I trust you will have an opportunity for some small pleasures in these dire times. I wish you peace.
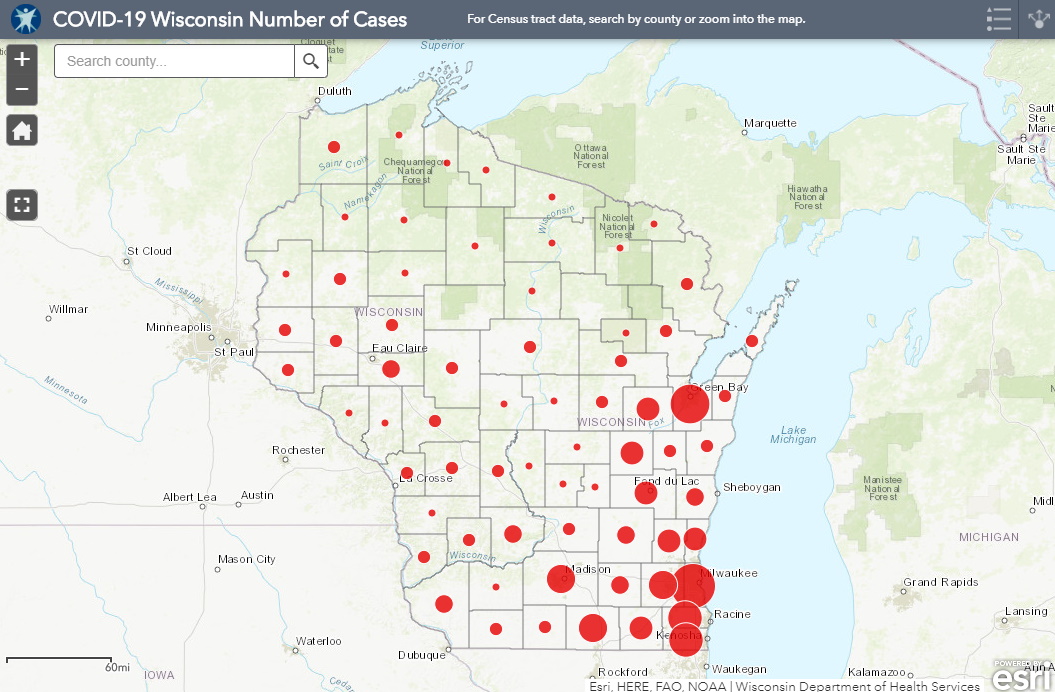
Two maps from the "Wild West" of Wisconsin and a cold, cold, cold hearted calculation.
Covid cases:
2016 election results by county:

The former map is likely to change because of the "Free Dumb" lawsuit.
Before the lockdown was invalidated by the Republican court, the number of cases in Wisconsin was 11,685, with 484 deaths, a death rate of 3.8%.
For people over 50, including my fellow baby boomers who love to congratulate themselves on saving the world, although they didn't, the number of cases in Wisconsin was 4,941. The overwhelming number of deaths was in people in this group, 425, giving a death toll of 8.6%.
Baby boomers are, despite how we congratulate ourselves on all of "our" liberal accomplishments, the demographic with the strongest support for the incompetent uneducated racist orange nightmare in the White House.
Trump won Wisconsin with a margin of about 22,000 votes. If 50% of those 22,000 were over 50 - this is a wild guess about the wild west - the number of cases required to halve Trump's margin, by resulting in the deaths of Trump voters over 50, is around 128,000 Covid patients over the age of 50.
We should assume that the majority of the people now partying in bars are Republicans, the "Free Dumb" crowd.
It is not likely that Wisconsin's voting will change entirely as a result of deaths by stupidity. On the other hand, deaths by stupidity certainly not going to help Republicans. It is also true that many patients who do not die will be nevertheless severely disabled. You never know, there may be some "Free Dumb" rednecks who will feel guilty for having killed grandma and grandpa in order to get a haircut and a beer.
They might stay home in the election or even change their vote.
Covid in Wisconsin
ERCOT Warns of Intensified Summer Supply Crunch For Texas Electricity Supplies.
I'm on the mailing list of Power, a trade magazine for the electric grid companies.
It's often an interesting read. I have a rather long post in process - which I may or may not finish - on the issue of process intensification, with a side bar on spinning reserve, the reserve power that grids keep running to deal with fluctuations in demand.
It appears that ERCOT, the grid that serves Texas is expected to operate at historical reserve lows this summer.
The news article is here: ERCOT Warns of Intensified Summer Supply Crunch
Some excerpts from the article:
ERCOT said its March 5–released final Seasonal Assessment of Resource Adequacy (SARA) for the upcoming spring season (March to May) and its preliminary assessment for the summer season (June to September) suggest the independent system operator (ISO), which manages about 90% of Texas’s electric load, faces markedly tighter supply scenarios this summer.
“Prior to each season, we consider a range of potential risks to determine whether there will be sufficient capacity to meet the expected peak load forecast,” said ERCOT President and CEO Bill Magness in a statement on March 5. “In all of the scenarios studied, we identified a potential need to call an energy alert at various times this summer.”
When ERCOT declares an alert, it “can then take advantage of additional resources that are only available during scarcity conditions,” the grid operator noted. “These resources include demand response products, resources that are normally set aside to provide operating reserves (including contracted load reduction from some industrial facilities), additional generation or imports from neighboring regions and voluntary calls for conservation by consumers...”
... The SARA also considers about 381 MW of typical maintenance outages, as well as forced outages of 3,845 MW, both based on data from June through September over the past three years. Other risks include low wind output, which would require securing about 3,959 MW in an extreme scenario.
As ERCOT explained, the SARA focuses on the availability of sufficient operating reserves to avoid emergency actions such as deployment of voluntary load reduction resources. “It uses an operating reserve threshold of 2,300 MW to indicate the risk that an Energy Emergency Alert Level 1 (EEA1) may be triggered during the time of the forecasted seasonal peak load,” it said. “This threshold level is intended to be roughly analogous to the 2,300 MW Physical Responsive Capability (PRC) threshold for EEA1..."
I've added the bold in this excerpt.
In the post I've been writing, which began with commentary on a scientific paper on electrochemical carbon capture and went somewhere else, I have an explanation of the real costs of spinning reserve, which explains why Germany and Denmark have the highest electricity prices in the OECD, despite all this mindless bullshit about how low the prices are for wind and solar energy.
Here's another article from the current issue of Power:
Nuclear Power Plants Set Performance Records in Spite of Pandemic
...and another...
Nuclear Reactor with 3D-Printed Core Slated for Operation in 2023
It's always a good thing when you know something about which you're talking. I'm not in the power industry, but I read this trade publication from it anyway.
Video Presentation on the Utility of Household Fabrics for Face Masks.
Here is the link for ACS Webinars: ACS Webinars
Although I watched it using my membership, I think for the next 21 hours, the webinar below should be available to the public free of charge if one registers.

(Afterwards, an ACS membership is required to watch it.)
It is a nice talk on the efficiency of household fabrics, as well as the approach to reusing N95 face masks, by sterilizing them.
It may be useful to watch.
There are actually two lectures, one by a scientist at Argonne National Lab/University of Chicago, and another by a materials science professor at Stanford.
They are not tremendously technical, but a small amount of technical knowledge may be helpful.
If you have time, check it out.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,526