NNadir
NNadir's JournalThe Cost of Electrolytic Hydrogen from Various Sources of Primary Energy.
The current issue of Industrial Engineering and Chemistry Research is the "Hydrogen Economy Issue." It contains 12 papers; most issues of this journal, which I read, regularly have more than 30 to 40 papers. This I think is a good thing. The hydrogen cheers over the last half a century or so that I've been hearing them are all paeans to wasting energy.
Hydrogen on this planet is not a primary source of energy; never has been; never will be.
The fantasy that runs around is that we'll all have "renewable hydrogen" made with so called "renewable energy" but for 50 years of wild cheering, "renewable energy" remains a trivial form of energy; I sometimes doubt that all the wind and solar facilities on this planet could run all the servers and computers dedicated to saying how great it is.
There are many reasons besides the thermodynamic losses that consumer hydrogen is a bad idea; the material issue of hydrogen embrittlement in materials science is just one example. The ridiculously low viscosity is another, as is the ridiculously low critical temperature.
Almost all of the world's hydrogen today, as his been the case for well over a century, is made by the steam reforming of dangerous fossil fuels. Electrolytic hydrogen has risen in recent years to about 4% of the world's hydrogen, but it's still a minor contributor and the hysteresis associated with shut down and restart of electrolytic cells means that it is particularly a bad idea to shut it down when the sun goes down and/or the wind isn't blowing.
Nevertheless the fantasy never goes away, does it?
The opening article of the current 12 paper issue of Industrial Engineering and Chemistry Research, Vol 61, Iss 18 is this one:
The Hydrogen Economy Preface Lourdes F. Vega and Sandra E. Kentish Industrial & Engineering Chemistry Research 2022 61 (18), 6065-6066.
I think it's open sourced.
First a little poetic color about defining the form of primary energy wasted to make hydrogen:
Most countries foresee a transition from fossil fuel-based hydrogen supplies to green hydrogen as the technology develops. However, in 2020, only 2% of hydrogen produced globally was from electrolysis, with 76% produced from natural gas and 22% through coal gasification. (9) This hydrogen was primarily used in oil refining, to produce ammonia as a fertilizer ingredient, or for methanol production, (9) rather than as an energy carrier.
Now the cost:
There you have it folks. So called "green" hydrogen is about 800% more expensive than dangerous natural gas, which should not be surprising since overall on this planet, hydrogen is made by burning dangerous natural gas.
I know we all like to play pretend, and not hear what we don't want to hear, but reality bites.
I have hopes for thermochemical hydrogen as a captive intermediate, but no, that's not going to happen by trashing thousands of square miles with mirrors to make in flight bird fryers while playing Archimedes at Syracuse. It can only be done with reliable and sustainable energy and solar thermal ain't it.
Have a nice evening.
A Biomarker For Intellect?
Some years ago, I made fun of the concept of the measurement of "intelligence" at another website:
A Note on This Race and IQ Business.
I came across this article in one of my news feeds this morning:
Mila M. Paul, Sven Dannhäuser, Lydia Morris, Achmed Mrestani, Martha Hübsch, Jennifer Gehring, Georgios N. Hatzopoulos, Martin Pauli, Genevieve M. Auger, Grit Bornschein, Nicole Scholz, Dmitrij Ljaschenko, Martin Müller, Markus Sauer, Hartmut Schmidt, Robert J. Kittel, Aaron DiAntonio, Ioannis Vakonakis, Manfred Heckmann, Tobias Langenhan, The human cognition-enhancing CORD7 mutation increases active zone number and synaptic release, Brain, 2022;, awac011.
I actually don't have access to this journal for one reason or another, but the Abstract gives the flavor by noting that you and I are very much like flies:
To this end, using protein expression and X-ray crystallography, we solved the molecular structure of the Drosophila C2A domain at 1.92 Å resolution and by comparison to its mammalian homolog ascertained that the location of the CORD7 mutation is structurally conserved in fly RIM. Further, CRISPR/Cas9-assisted genomic engineering was employed for the generation of rim alleles encoding the R915H CORD7 exchange or R915E,R916E substitutions (fly numbering) to effect local charge reversal at the 310 helix. Through electrophysiological characterization by two-electrode voltage clamp and focal recordings we determined that the CORD7 mutation exerts a semi-dominant rather than a dominant effect on synaptic transmission resulting in faster, more efficient synaptic release and increased size of the readily releasable pool but decreased sensitivity for the fast calcium chelator BAPTA. In addition, the rim CORD7 allele increased the number of presynaptic active zones but left their nanoscopic organization unperturbed as revealed by super-resolution microscopy of the presynaptic scaffold protein Bruchpilot/ELKS/CAST.
We conclude that the CORD7 mutation leads to tighter release coupling, an increased readily releasable pool size and more release sites thereby promoting more efficient synaptic transmitter release. These results strongly suggest that similar mechanisms may underlie the CORD7 disease phenotype in patients and that enhanced synaptic transmission may contribute to their increased cognitive abilities.
There's that good ole' IQ again...
Maybe you want to tell me that you absolutely must have the R844H CORD7 mutation, just like the flies.
Don't get all worked up, maybe you don't want this mutation. It's associated with visual difficulties:
Samantha Johnson, Stephanie Halford, Alex G Morris, Reshma J Patel, Susan E Wilkie, Alison J Hardcastle, Anthony T Moore, Kang Zhang, David M Hunt, Genomic organisation and alternative splicing of human RIM1, a gene implicated in autosomal dominant cone-rod dystrophy (CORD7)☆, Genomics, Volume 81, Issue 3, 2003, Pages 304-314.
I do have access to this one. From the introduction:
Cone-rod dystrophy is characterized by the early loss of visual acuity and color vision, followed by night blindness and peripheral visual field loss [1]. Autosomal-dominant, X-linked, and recessive modes of inheritance have been described, and recent genetic studies have implicated a variety of different genetic loci in the etiology of this set of heterogeneous disorders, although the disease loci that underlie most of the cone and cone-rod dystrophies have yet to be identified.
An autosomal-dominant cone-rod dystrophy, CORD7, was originally mapped in a four-generation British family to a region of chromosome 6q14 that is flanked by markers D6S430 and D6S1625 [2]. This localization for CORD7 overlaps or is adjacent to the map locations of a number of other retinal disorders. These include, in the overlapping category, a recessive form of retinitis pigmentosa (RP25) [3], Leber congenital amaurosis type 5 (LCA5) [4], and a dominant drusen and macular degeneration [5] and in the nonoverlapping category, North Carolina macular dystrophy (MCDR1) [6], a dominant Stargardt-like disease (STGD3) [7], [8], and a dominant macular atrophy [9]. STGD3 has recently been shown to arise from mutations in ELOVL4, a gene encoding a protein with a possible activity in the biosynthesis of very long-chain fatty acids [10].
The onset of reduced color vision and visual acuity in affected members of the CORD7 family varies between the ages of 20 and 40 years [2]. As the disorder progresses, difficulties of seeing in bright light become apparent, and one individual also reported visual problems in dim light. At the onset of symptoms, retinal pigmentary changes are already present around the fovea, which develops into macular atrophy. Electrophysiological examination shows that scotopic rod responses in patients with advanced disease are barely detectable, and all cone responses are severely attenuated but with no change in implicit time. Pattern electroretinogram is extinguished in keeping with the severe macular dysfunction [2].
Our strategy for identifying the disease gene has been to prioritize the screening of candidate genes on the basis of function and pattern of gene expression. Three loci were considered excellent candidates, the interphotoreceptor matrix proteoglycan gene, IMPG1 [11], atypical myosin VI, MYO6 [12], [13], and Rab3-interacting molecule, RIM1 [14]...
If however, you have night blindness - my wife actually has a mild case of this - you can always tell me how much smarter you are than I am.
I won't argue.
See ya'...
Dawn Powell lived in Shelby, Ohio...
...wrote the novel "The Locusts Have No King," died in obscurity, donated her body to science, which, after use, an attempt was made to return it to the executor of her will, who refused it, whereupon it was buried in a Potter's Field.
There is no reason that you would need to know that, no reason you should care, but as you have read this, now you do in the unlikely case it ever comes up in conversation.
Life is beautiful and then you die.
In Memoriam: Babatunde Ogunnaike, 1956-2022
Back when my son was touring colleges, having heard from a friend that the University of Delaware gave great scholarships to excellent students and that the Materials Science Engineering Department was pretty good we visited the school to attend an engineering open house. (My son applied there and was accepted, but in the end the scholarship offered was not quite as good as many others),
I don't remember much about the tour other than the very moving speech that Dr. Babtunde Ogunnaike gave to the prospective engineering students around the theme of "If not you, then who?"
I did not know much about his work, but of all the speeches in college tours one hears, that one stuck in my mind.
He apparently has died:
In Memoriam: Babatunde Ogunnaike, 1956–2022 Phillip E. Savage Industrial & Engineering Chemistry Research 2022 61 (16), 5365-5365.
Some text:
Tunde was an international leader in chemical engineering and the process systems engineering community. During his tenure as Associate Editor, Tunde handled thousands of manuscripts dealing with process control, design, operations, monitoring, and broader systems engineering topics. His service to the journal was exceptional, and his impact upon I&EC Research and chemical engineering as a field is immense. Because of his many professional accomplishments and contributions, Tunde was elected to the National Academy of Engineering in 2012.
Before joining the faculty at the University of Delaware, Tunde served on the faculty at the University of Lagos for six years and at the DuPont Co. for 13 years. This international and industrial experience gave Tunde a broad and unique perspective of his field, which served the journal and its authors well.
Those of us who were fortunate enough to work with Tunde will always remember him as a delightful colleague, keen wit and intellect, warm friend, generous mentor, and consummate professional. He will be greatly missed. A special issue of this journal will be published in Tunde’s memory, celebrating his legacy, and recounting his accomplishments in more detail...

LaFeO3 Catalysts for the Oxidation of Nitrogen for Electrochemical Production of Ammonia from Air.
Recently in I have been considering the energy requirements for the zero discharge supercritical desalination of seawater, modeling the urban and agricultural demand for water in California as a tool for this relatively simple calculation to which I've chosen to add some level of complexity.
California and I got divorced nearly thirty years ago, but in my own way, I still love her, but some of what I loved was its spectacular ecology and vistas, many of which have been trashed for access to water and for access to industrial energy.
I view the water problem as a direct opportunity to restore, at least partially, trashed ecosystems.
Supercritical water is a very high energy substance, at temperatures in excess of 373°C, and pressures in excess of 22.1 MPa, and as I envision a process for its generation for the purpose of desalination, the side product would be continuously and reliably produced copious electricity. Electricity is, of course, a thermodynamically degraded form of energy, particularly when it is generated by the combustion of dangerous fossil fuels, as increasingly it is. This is true even for relatively efficient systems, for example combined cycle plants fueled by dangerous natural gas. The thermodynamic degradation of electricity is even worse when attempts are made to store in a chemical form, as in batteries or, for another example along the same terrible idea, as electrolytic hydrogen.
A caveat to the previous paragraph is that when electricity is generated in a process designed to capture entropic losses in heat engines as a form of exergy, which electricity is at least indirectly, it can actually improve thermodynamic efficiency, at least in the case where the electricity is utilized continuously at the time it is generated, foregoing energy storage. I am thus always interested in electrochemical processes where the purpose of the electrochemistry is not to store it, for example as hydrogen, but to rather in processes where it's use is either essential or where the alternatives are even more thermodynamically or materially questionable than electricity itself. Examples of such processes are the Hall-Heroult process by which all the world's aluminum metal is prepared from alumina, Al2O3, an extremely refractory process, as well as the FFC Cambridge process for the preparation of titanium and other metals. I also have interest in the electrochemical process for the preparation of elemental carbon about which I've written elsewhere in this space: Electrolysis of Lithium-Free Molten Carbonates. (In envision this process as a means to replace "Green" electrodes the Hall-Heroult process, which are made from petroleum coke, as well as FFC electrodes.) This electrochemistry, if applied to carbonates derived from air or seawater captured carbon dioxide might also displace – with some research – the carbon content in steel, thus usefully sequestering the element, essentially indefinitely.
It is because of this consideration, the need to recover exergy from thermal desalination via electricity generation that the following paper caught my attention:
Coupling of LaFeO3–Plasma Catalysis and Cu+/Cu0 Electrocatalysis for Direct Ammonia Synthesis from Air, Yi Cui, Hui Yang, Chengyi Dai, Pengju Ren, Chunshan Song, and Xiaoxun Ma Industrial & Engineering Chemistry Research 2022 61 (14), 4816-4823.
The synthesis of ammonia, on which the current world food supply depends, is responsible, depending on one's source, for 1 to 3 percent of the world's energy supply. Currently the world demand for energy is on the order of 600 EJ/year; the Covid lockdowns in Shanghai and other Chinese cities may keep the demand below 600 EJ in 2022 as worldwide Covid lockdowns seems to have done in 2020. This means that the demand for Ammonia represents between 5 and 15 EJ per year, somewhere between the entire energy demand of Great Britain and that of Germany, the latter being largely dependent on Russian fossil fuel sources.
Ammonia is currently industrially synthesized using the Haber-Bosch process, which relies on the high temperature, high pressure catalytic hydrogenation of nitrogen gas. Despite some rather dubious enthusiasm for the fantasy that hydrogen might someday be produced by so called "renewable energy" via the thermodynamic and material nightmare of electrolysis - hydrogen is a currently a very dirty fuel, not that much of of it actually utilized as a fuel except in exotic situations hyped by people who seem not to understand the laws of physics well or at all. I noted elsewhere what the sources and uses of hydrogen are: The current sources and uses of hydrogen.
Here is a graphic from that post:
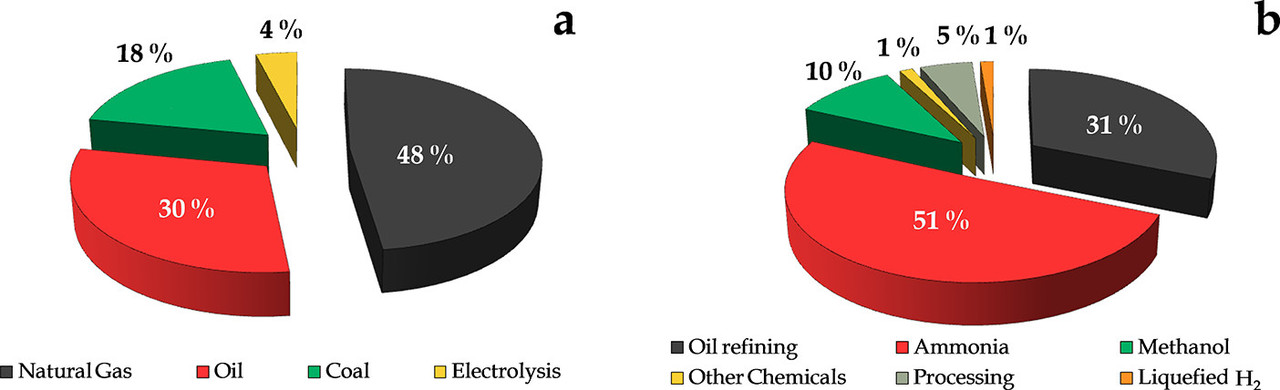
The caption:
Here is the graphic's source: Progress on Catalyst Development for the Steam Reforming of Biomass and Waste Plastics Pyrolysis Volatiles: A Review Laura Santamaria, Gartzen Lopez, Enara Fernandez, Maria Cortazar, Aitor Arregi, Martin Olazar, and Javier Bilbao Energy & Fuels 2021 35 (21), 17051-17084.
As we can see from the above graphic, hydrogen is a dirty fuel as it is at a minimum 96% dependent on fossil fuels dangerous fossil fuels for production - this at a thermodynamic loss - and depending on the source of electricity, which is also largely dependent on dangerous fossil fuels it may be even more dependent on them than is immediately obvious. (Electrolytic hydrogen is generally produced as a side product from the manufacture of elemental chlorine.)
The Dai and Ren paper under discussion is about the plasma production of fixed nitrogen followed by a electrochemical step. Every winter I attend lectures - except recent winters, lately they've been on line - at the Princeton Plasma Physics Laboratory. It would be disingenuous of me to criticize the hopes for fusion energy to which that national laboratory is mostly dedicated, but to the extent that it may become available (I won't object) or viable, it will be already too late to address the climate disaster, any more than the idiot fantasies of the members of Greenpeace spin about so called "100% renewable energy" "by such and such a year," even as people are dying today from extreme May heat in India and Pakistan.
Today.
Now.
This is why fusion energy will be too late because the climate disaster is underway now; it's on going.
Many of the winter lectures at the Princeton Plasma Physics labs are about all the wonderful things that plasma, a state of matter often referred to as the "4th State of matter" (there are arguably more than 4 states), can do besides run a fusion reactor. I do not recall that any I've attended have focused on the fixation of nitrogen, but in any case were such a lecture to be offered, it would not be about anything new. The nitrogen problem is an old one and was recognized as such - as a cause of soil depletion that, among many other things, famines for instance, that contributed to the outbreak of the American Civil War - early in the history of Chemistry. With the final acceptance of molecular theory, efforts got underway to try to make ammonia once people understood what it was. Mostly they failed. Early attempts to industrially fix nitrogen before the invention of the Haber-Bosch process that were marginally successful, albeit incredibly expensive, utilized electric spark methods; electric sparks are plasma. This is all described in a book I cannot recommend enough Vacslav Smil's Enriching the Earth, Fritz Haber, Carl Bosch, and the Transformation of World Food Production. It is all about the work on the synthesis of ammonia from hydrogen and nitrogen that brought Haber the Nobel Prize and allowed Germany to fight the First World War despite the fact that British blockades prevented Germany from importing Chilean "Salt Peter," (potassium nitrate). This work also allowed humanity to grow beyond Malthusian limits to a population now just shy of 8 billion.
There are more modern methods than spark generation to produce nitrogen plasmas, there are a number of analytical chemistry instruments, for example, that work on "corona discharge" technology involving activated nitrogen, but none of them are used to fix nitrogen at anything competitive with the cost of the Haber Bosch process. The authors of the paper under discussion propose a new catalyst for the production of activated nitrogen plasma, lanthanum ferrate. Whether it would be competitive is not for me to say, but frankly, I'm rather disinterested in the plasma portion of this paper, but am very interested in the electrochemical portion.
The reason is this: At high enough temperatures - especially under pressure - nitrogen burns to form nitrogen oxides; nitrogen oxides are an important component of air pollution. The brown color of smog is, in fact, the color of NO2 gas.
All combustion cycles driven by dangerous fossil fuels produce nitrogen oxides, both in automobiles and trucks, particularly where diesel engines are involved but also in spark (Otto) engines, as well as in power plants. The purpose of catalytic converters - which are never 100% efficient - and the addition of urea to diesel exhaust, the so called "SCR," approach - is to remove nitrogen oxides. Jet engines, which are known as Brayton cycle devices produce nitrogen oxides as do the combustion chambers of dangerous fossil fuel heated Rankine cycle type power plants.
In the presence of water the nitrogen dioxide, the aforementioned NO2, disproportionates into NO gas and nitric acid; the NO gas is rapidly oxidized back to NO2 by oxygen. The net reaction for the formation of nitric acid, a component of "acid rain," in which NO gas is catalytic, is 4NO2 + 2H2O + O2 ? 4HNO3. This is the basis of the Ostwald process for the production of nitric acid from ammonia. The reaction between ammonia is the basis of the widely utilized fertilizer ammonium nitrate which the terrorist Timothy McVeigh used to blow up the Murrah building in Oklahoma. (No one ever called for the banning of agriculture because ammonium nitrate can be diverted to weapons of mass destruction.)
For reasons of high thermodynamic efficiency, the process I imagine for for supercritical water desalination begins not with the heating of seawater, but rather the heating of pressurized air, precisely the conditions under which nitrogen "burns." For a long time I thought about how to address the resultant nitrogen oxides by utilizing approaches to catalysis rather like those utilized in Otto and Diesel internal combustion engines, but this is unsatisfactory in the sense that it wastes fixed nitrogen which takes energy to make and is thus a useful material. One can imagine a closed system under these conditions in which the electrochemical reduction of nitrate and nitrite, formed respectively by the addition of water to nitrogen oxides might be reduced to a useful product.
Shortly I will quote the paper's introduction.
Before doing so, I'd like to make a brief note on the nature of citing papers in connection with a personal issue. If one cites a paper, it does not follow that one agrees either with the contents of the paper or even with the paper's goals. I mention this because recently an abysmal idiot with whom I have a rather unhappy relationship in this space punctuated by ignoring his or her ignorance on and off, commented in one of my posts that I must have Alzheimer's disease because I was mocking a paper I cited in the OP to, um, mock. One sees these sorts of things, and one really doesn’t believe it. Regrettably the comment was removed for violating DU rules, which is sort of sad (but understandable) when someone, not me, alerted on it. Personally I guiltily enjoy it when idiots display idiocy.
The paper in the post was about the issue of flowback water used to frack for dangerous natural gas - a process I oppose in all manifestations - and which the abysmal idiot in question apparently needed to defend, since so called "renewable energy" depends wholly and totally on access to dangerous natural gas and/or other dangerous fossil fuels.
I mention this because, although I think that in many ways, this is a fine paper, there are several points in the introduction with which I decidedly disagree although I suspect that one of them is merely an obeisance paid to what is becoming a culturally universal faith, albeit it one hardly grounded in reality.
The quote from the introduction:
The electrochemical NH3 synthesis from N2 and H2O using renewable energy sources such as solar and wind energy has emerged as a potential alternative in recent years. (11?13) Compared with the H–B process, significant advantages could include mild reaction conditions, no direct CO2 generation, operability for smaller scale production, and low equipment cost. (14?16) However, because of the extremely low solubility of N2 in H2O and its high triple bond energy (948 kJ mol–1), activating N2 in electrochemical systems is challenging, (17,18) and only low yields (less than 0.1 mg h^(–1)) and faradaic efficiencies for NH3 (less than20%) have been achieved. (12,19)
However, the solubility of nitrate (NO3–) and nitrite (NO2–) in H2O is nearly 40000 times that of N2, ensuring sufficient amounts of N source in the electrolyte. In addition, the energy required for dissociation of the N–O bond is only 21.7% of that of the N2 triple bond. (20) As a result, NOx– can be easier reduced to NH3 than N2. (21?24) Zhang et al. proposed a N2 fixation strategy of oxidation and reduction in electrocatalytic systems. (25) In this work, the yield of NO3– in the electrocatalytic N2 oxidation process is too low to provide an N source for the reduction process directly. Nonthermal plasma (NTP), characterized by its large number of high-energy electrons and a low gas temperature, (26) can promote the activation of N2 under mild conditions. (27,28) Therefore, NTP is applied in the N2 oxidation process in the coupling strategy. (29) Generally, the efficiency of N2 fixation in the NTP can be improved by using a catalyst. (30?32)
Perovskites are used in various reactions, (33?36) especially for oxidation reactions such as the oxidation of nitrogen oxide (35,37,38) and carbon monoxide, (39,40) because of their excellent thermal stability, electronic structure, ionic conductivity, electron mobility, and redox behavior. Consequently, perovskites are expected to display excellent performance in NOx production. Copper-based catalysts are widely used in the electrochemical reduction of NOx–. (41?44) However, copper (Cu) could be deactivated due to excessive adsorption of NO2– on the surface during the reduction of NO3–. Therefore, copper(I) oxide (Cu2O) with special electron-donating characteristics needs to be introduced in environments with high concentrations of NO3– and NO2–. (45)
Considering these aspects, we present an N2 fixation strategy to produce NH3 based on the coupling of N2 oxidation in NTP and electrochemical NOx– reduction in H2O. Briefly, using LaFeO3 as the catalyst, N2 and O2 in the air are first oxidized in NTP to NOx, which enters H2O to produce NO3– and NO2–. Then the NOx– species are electrochemically reduced to NH3 over a Cu+/Cu0 catalyst with considerable activity and excellent faradaic efficiency (FE).
My first objection to the statements herein is that ammonia is a terrible fuel as a "hydrogen carrier" because it is even more dangerous than hydrogen itself; it's only advantage being that it is easily liquified. Many chemists have worked with liquid ammonia and they need to be trained to do so. It boils readily at room temperature releasing a caustic gas that will quickly either blind people painfully, badly damage their lungs and/or kill people. Although it avoids the huge energy penalty associated with the liquefaction of hydrogen that makes adds to the waste of energy and environmental degradation that hydrogen production entails, and is slightly less dangerous from an explosion and leak perspective from hydrogen resulting from hydrogen embrittlement of metals, it is no less unacceptable than hydrogen. The idea of using hydrogen or ammonia as a consumer product borders on insane in my view, although neither of these dumb ideas ever seem to go away.
My second objection is the statement that so called "'renewable energy sources such as wind and solar has emerged as a potential alternative in recent years." This kind of statement is often included in scientific papers these days but on reflection, it's not even close to being "science," so much as a quasi-religious genuflection at the nonsense belief that solar and wind are meaningful and/or sustainable forms of energy. They are not. They have failed, and failed miserably to address climate change and all the wishful rhetoric to the contrary is clearly nonsense. After more than 50 years - more than half a century - of cheering for these forms of energy they produced, as of the 2021 Edition of the International Energy Agency's World Energy Outlook, just 10.4 Exajoules out 587 Exajoules consumed by humanity in 2020, this a Covid lockdown year in which for the first time in recorded history energy demand fell, albeit clearly temporarily. The material and land requirements of the solar and wind industry render them immediately unsustainable, particular with matter and energy wasting systems like the batteries people fall all over themselves to praise in contempt for all future generations. We have spent well over 3 trillion dollars on this stuff in this century for no result other than this:
May 06: 419.61 ppm
May 05: 419.68 ppm
May 04: 421.33 ppm
May 03: 420.48 ppm
May 02: 420.99 ppm
Last Updated: May 7, 2022
Recent Daily Average Mauna Loa CO2
These things are facts. Facts matter, no matter how much wishful thinking and squirming they may produce.
One advantages of the side product of supercritical water desalination being excess electricity is that it will allow for the restoration of wilderness in California - over 1500 square miles of wilderness as of this writing - out of the wind industries industrial parks and allow for the dismantling of that horrible in flight bird fryer at Ivanpah. It is the land requirements for so called "renewable energy" that makes it unsustainable; it is the material (mining) requirements that makes the word routinely attached to it, "renewable" absurd and frankly, a lie.
Despite these statements in the introduction, I think this paper is a valuable one because it addresses the high energy cost of ammonia production even if the Haber Bosch process has approached it's theoretical limits. Most of this energy, as the paper notes, is connected with the need to overcome the energy barrier of breaking NN triple bonds. This is nicely shown by the energy level diagram for the nitrogen and nitrogen-oxygen species contained in the paper:

The caption:
It is worth noting that in this energy diagram the activation energy for the production of monoatomic N, reflecting the energy cost of breaking the NN triple bond, is similar in scale to that of breaking the bond in the Haber-Bosch process, which also involves surface chemistry - the catalyst. The N2* refers to the non-thermal plasma. In the process I imagine for SCW desalination the gas phase energy is a side reaction. This said, if the goal of the process is to produce ammonia, however, one can also irradiate the air with gamma rays or x-rays, a source of which might be isolated fission products.
I note that lanthanum is a fission product, but all of its radioactive isotopes are short lived, with the exception of that found naturally, La-138, which has a half life of 0.1 trillion years. After a few weeks, allowing for the decay of La-140 (half-life around 1.68 days) lanthanum isolated from used nuclear fuel would be less radioactive than natural lanthanum, since La-138 does not form to an appreciable extent in nuclear fuels as it is neutron poor. Thus one would need another element as a gamma source to produce N2* radiolytically.
A note on NO: In the presence of oxygen and water, NO gas can react to form nitrous acid, HNO2, as well as nitric acid HNO3. Absorbed into water in a system in which ammonia is being generated, ammonium nitrite will form. Ammonium nitrite is unstable and spontaneously can decompose to nitrogen gas and water. Thus the reaction efficiency is somewhat lower than it is for NO2's reaction to form nitric acid. As the Oklahoma City tragedy perpetrated by the early right wing terrorist Timothy McVeigh showed, ammonium nitrate can also explosively decompose if activated, which McVeigh did by diverting a dangerous fossil fuel, diesel fuel, to a weapon of mass destruction, but in general ammonium nitrate in contrast to ammonium nitrite can be handled and shipped as it often is. It is an important fertilizer on which our food supply depends. Under the right conditions, this electrochemical process can make it without the high temperature and high pressure systems associated with the long practiced industrialized Haber Bosch process.
I have ignored much of the interesting content of the paper in this post, but it's a good one, I think.
From the author's conclusion:
I will be celebrating Mother's Day today with the woman of my dreams, who chose to allow me two magnificent sons. I am grateful to have her as a friend, a lover, a friend, and as the parent of my boys. If you are celebrating Mother's Day in some capacity, I wish you a happy one; otherwise I wish you a wonderful afternoon.
As we prepare ourselves for the Supreme's theocracy, reference to other human sacrifice for "faith."
I'm not crazy about the New York Times "science" reporting, but this article caught my eye:
They Thought the Skulls Were Murder Victims’. They Were Off by Centuries.
It's probably behind a fire wall - in spite of my better judgement I subscribe to the NY Times - but an excerpt:
The police started an investigation, believing it was a crime scene of migrants killed near the border with Guatemala, where gang violence is commonplace.
Indeed, it was a crime scene. Just not one that occurred recently.
Last week, 10 years after the discovery, the authorities said in a statement that they had determined the skulls were from sacrificial killings between A.D. 900 and 1200.
“We have already learned a lot of information,” Javier Montes de Paz, an archaeologist who analyzed the bones, said in a news conference on April 11. “But it’s also important to note: What were those craniums doing in that cave?”
Researchers at the National Institute of Anthropology and History analyzed marks on the bones and determined that the deaths had happened centuries ago. Such marks would appear only after “a lot, a lot of time” had passed, Mr. Montes de Paz said.
The researchers found that the victims had been beheaded, that most of the bones were from female victims...
I added the bold.
Apparently 5 members of the Supreme Court worship tissue in women's bodies so much that they are willing to sacrifice the women themselves, raped women, poor women, just women, and the reason they are willing to make this sacrifice is because while the 1st Amendment precludes Congress from establishing a state religion, they feel the Supreme Court is allowed to establish a State religion, apparently one not all that distant in its view of women than that practiced in Mexico about 1000 years ago.
In this country, the enemy of the Constitution is the group of illegitimate thugs established on the Supreme Court by the racist, sexist thug Mitch McConnell, who violated the precedent set by none others than John Adams and Thomas Jefferson, when Jefferson and Congress accepted as perfectly legitimate the appointment (by Adams) of the precedent setting great Chief Justice John Marshall at the very end of Adam's term.
The idea that the modern day American Taliban - aka the "Republican" party - loves our country and its constitution is obscenely laughable.
We need to fight, and fight hard.
Interesting new lipophilic extractant for trivalent actinides.
Regrettably I don't have much time to discuss this paper that I just came across. For the essential reprocessing of used nuclear fuel, our last, best hope of doing anything about climate change, it is important to recover the minor actinides, specifically neptunium, americium and curium because of their very special nuclear properties, only one of which is for proliferation resistance.
I'm not a PUREX kind of guy, but currently the world's most common nuclear reprocessing is accomplished using this process or a variant thereof.
I'm an electrochemical separations kind of guy, and one interesting approach that I think needs advancement is electrochemically driven membrane separations, for which traditional extractants have some value in continuous flows in conducting ionic liquid membranes. (I see the world slowly moving in this direction.
The paper that caught my eye: is this one: Promising Lipophilic PyTri Extractant for Selective Trivalent Actinide Separation from High Active Raffinate Annalisa Ossola, Eros Mossini, Elena Macerata, Walter Panzeri, Andrea Mele, and Mario Mariani Industrial & Engineering Chemistry Research 2022 61 (12), 4436-4444
An excerpt:
This 370,000 MT of used nuclear fuel has tremendous clean energy value - the energy density of plutonium is 80 trillion joules per kg - without requiring additional mining, and consists of many extremely valuable components beyond the actinides, some of which are happily highly radioactive.
Here is the structure of this new interesting extractant which suggests many modifications that might make an ionizable form for ion transport:

The caption:
Nice actinide/lanthanide separation factors:

The caption:
We can save the world if we open our minds and stop chasing unicorns.
Esoteric but cool, in particular as the paper is out of an Italian institution and Italy is an officially anti-nuke country.
Happy Friday...
The turnaway study: Longitudinal study of the denial of abortion rights.
It was referenced in this weeks Nature.
The Turnaway Study
Factsheet: The Harms of Denying a Woman a Wanted Abortion
A Summary of Publications on The Turnaway Study
What is The Turnaway Study?
The Turnaway Study: Ten Years, A Thousand Women, and the Consequences of Having - or Being Denied - an Abortion
Before the Turnaway Study, there was little quality research on the physical and social consequences of unwanted pregnancy for women. Most of the research that did exist focused on whether abortion causes mental health problems such as depression and post-traumatic stress disorder, or alcohol and drug use. That body of work often used inappropriate comparisons groups—comparing, for example, women who obtain abortions with those who continue their pregnancies to term by choice—and used retrospective designs that depended on women’s reporting of pregnancies and abortions in hindsight. Such comparisons are inherently biased and paint a distorted picture of life following an elective abortion or pregnancy continuation.
Yes, science can weigh in on abortion law.
This is a "World View" article in Nature. It dates before the reactionary decision about to be announced by the Taliban dominated Supreme Court that was politicized by the racist sexist thug McConnell enabled by liars including Susan Collins.
Yes, science can weigh in on abortion law
Subtitle:
Diane Green Foster, Nature, November 16, 2021.
I believe it's open sourced, but here are some excerpts:
Yet some countries, such as the United States, Poland and Nicaragua, are making access to abortion more difficult. Restrictions are passed on the basis of ideology or political motives, without considering scientific evidence about their impact. Science might not be able to decide philosophical questions about when life begins or when the rights of a fetus outweigh the agency of the person whose body is necessary for its growth and development. But it can tell us how access to abortion is affected by its legal status, and about the consequences when abortion is inaccessible. Science should weigh in on the often quoted yet seldom tested slogans of the abortion debate, because people’s well-being is at stake.
Consider this argument: ‘One cannot ban abortion; one can only ban safe abortion.’ This can be tested. When abortion is illegal, pregnant people are more likely to resort to unsafe methods. But some circumvent the law in ways that are safe. Those with the means travel to places where abortion is legal, and others take safe medications, approved by the World Health Organization, to terminate their pregnancies outside the formal health system. In Latin America, where self-managed abortion is widespread, large decreases in mortality from unsafe abortion have been documented without widespread changes to restrictive abortion laws (see go.nature.com/3d6gspd).
But there’s another consequence that should be investigated — when people are unable to get a legal abortion, they are more likely to carry unwanted pregnancies to term. It is estimated that 70% of unintended pregnancies end in abortion in places where it is legal, compared with about 50% where it is not (J. Bearak et al. Lancet Glob. Health 8, E1152–E1161; 2020). I am a demographer who gathers data and creates quantitative models to assess how unintended pregnancies affect the well-being of women, children and families. My work shows that there are serious ramifications.
Most of my evidence is drawn from the Turnaway Study, which I led. My team and I followed almost 1,000 women for five years after they sought an abortion in the United States, comparing the health and socio-economic consequences of receiving an abortion or being denied one. We found serious physical health consequences from continued pregnancy and childbirth, including death. Women and their existing and subsequent children also experienced greater economic and other hardship when abortion was denied. Women were more likely to continue to be exposed to intimate-partner violence, less likely to have an intended pregnancy under better circumstances later, and less likely to achieve their own aspirations...
... Studies in other countries where abortion is legal — Colombia, Tunisia, South Africa and Nepal — have found that many women are turned away because of difficulties including a lack of trained clinicians and low knowledge of the law. Some get an abortion outside the legal system, sometimes with serious medical complications. Others plan to carry the pregnancy to term, and anticipate hardships. A woman in Tunisia remarked that she did not have clothes for a newborn. “Four children, and a fifth one on top! Where are we heading this way? Poverty and tyranny” (S. Hajri et al. PLoS ONE 10, e0145338; 2015). An 18-year-old in Colombia who would not be able to continue her studies once she had a baby said: “I will no longer be able to be young” (T. DePiñeres et al. Reprod. Health 14, 133; 2017).
There is much more science to be done on abortion access. What is the impact of gestational limits? Who crosses borders to get care? What information, support and services help people to use abortion medications safely...
File for "very long term," zircon supported catalysts for the transfer hydrogenation of bicarbonate.
In general, I oppose biofuels because of the massive destruction of critical ecosystems, as I remarked recently in a post here: The Very Stable Genius of Biofuels.
Nevertheless, they exist and in one case, the case of biodiesel, they have led to a glut of the simplest triol glycerol.
It does seem to me that algal biodiesel might have some potential to be marginally sustainable, possibly as a side product, as a tool to recover phosphorous from eutrophic zones, particularly in the case where waste or deliberate heat was sustainably available. In this more sustainable case, glycerol would still represent a glut.
I'm not going to spend very much time at all on the paper I'll reference here, if for no other reason that transfer hydrogenations have always struck me as cool. Here it is: Ru/ZrO2 as a Facile and Efficient Heterogeneous Catalyst for the Catalytic Hydrogenation of Bicarbonate Using Biodiesel-Waste Glycerol as a Hydrogen Donor Wubin Yan, Binbin Jin, Jiong Cheng, Xiaoyu Shi, Heng Zhong, and Fangming Jin ACS Sustainable Chemistry & Engineering 2022 10 (17), 5374-5383.
This brief note is a placeholder, should it ever be possible to make sustainable biodiesel - it will never be as clean a diesel fuel as DME but it may have a role to increase lubricity - something which is currently not viable from an environmental standpoint.
From the introduction to the paper:
Recently, hydrogenation of CO2 using biomass as the hydrogen donor emerged as a novel method. (4,5) Compared to the traditional hydrogenation process, this indirect hydrogenation strategy could achieve desirable CO2 conversion with a promising carbon-negative effect by making full use of renewable and naturally abundant biomass as the reductant. (6) Our group has investigated the hydrogenation of HCO3– into formate under hydrothermal conditions by using the glycerol as a green hydrogen source instead of gaseous hydrogen. (7) As a main side product of the biodiesel manufacturing, glycerol has been applied as an attractive bio-based platform substrate to the production of high-value chemicals. (8) Hydrogenation of HCO3– with glycerol shows an advantage on thermodynamics because glycerol can significantly lower the ?Gaq? for the hydrogenation of HCO3– compared with the direct hydrogenation. (9,10) In addition to providing hydrogen for HCO3– hydrogenation, the glycerol itself can be converted into lactic acid, achieving the synergistic conversion of glycerol and HCO3–. However, the quite limited conversion efficiency urges us to search for suitable catalysts.
Homogeneous catalysts, such as water-soluble iridium N-heterocyclic carbene catalysts, have been studied for the reduction of HCO3– with the participation of glycerol. (9) Although homogeneous catalysts can achieve a relatively high TOF value, the difficulties in separating and recovering them from the reaction system limit their application. Additionally, the stability of these homogeneous catalysts at a high reaction temperature is also one limit. By contrast, heterogeneous supported catalysts may be more desirable due to their better recoverability and stability. To strip hydrogen from the biomass and transfer hydrogen from biomass to active sites, the capacity of enhancing the hydrogen transfer in the whole conversion process is crucial for the catalyst to achieve efficient CO2 conversion using biomass as the reductant. Considering the significant advantages of noble metal in hydrogenation and dehydrogenation, noble metals were selected as the active components for the target catalyst...
I have time to post only one graphic from the article, a graphic on the mechanism:

The caption:
The products are lactate - utilized in certain biopolymers used widely in medical applications - formate and dihydroxyacetone (which rearranges to give lactate.)
Cool paper. Like I said, I like transfer hydrogenations.
Ruthenium is a relatively rare and expensive metal, but it can be recovered from used nuclear fuel in significant quantities as it is a prominent fission product. Zirconium, the support, which is less rare is also a fission product as well as a widely used structural component of nuclear reactors.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,577