NNadir
NNadir's JournalWe are about to have a President, Mr. Joe Biden. So why?
How is it that we have so many posts on DU discussing a lump of shit being loaded on a plane to head for a swamp?
Truth is the daughter of time, not of authority.
-Francis Bacon, as quoted by Dmitri Volkogonov, in Stalin, Triumph and Tragedy
Joe Biden names top geneticist Eric Lander as science adviser
From Nature News: Joe Biden names top geneticist Eric Lander as science adviser Nidhi Subbaraman & Alexandra Witze Nature News January 16, 2021.
It should be open sourced.
Some excerpts:
Many scientists have long called for the OSTP director to be raised to a cabinet-level position. “Having science elevated to its rightful place in the administration seems to me a very positive step,” says Harold Varmus, a cancer researcher at Weill Cornell Medicine in New York City and a former head of the US National Institutes of Health (NIH). “I think it marks a very important moment in the history of science in the government.”
“It signifies the importance of who will be in the room when decisions are being made,” says Roger Pielke Jr, who studies science policy at the University of Colorado Boulder.
Lander was a key figure in the Human Genome Project — the race to sequence the human genome, which ended in 2003 — and is the president and founding director of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. He will be the first biologist to run the OSTP.
Between 2009 and 2017, he co-chaired the President’s Council of Advisors on Science and Technology (PCAST), an elite panel that advises the US president. Among the PCAST reports issued during Lander’s tenure were some dealing with energy, climate change and vaccine response in the face of pandemic influenza...
… Nobel laureate Frances Arnold, a bioengineer at the California Institute of Technology in Pasadena, and Maria Zuber, a geophysicist at MIT, will co-chair PCAST under Biden. Alondra Nelson, nominated to be deputy director for science and society at the OSTP, is a social scientist at the Institute for Advanced Study in Princeton, New Jersey, who studies genetics, race and other societal issues.
“These are excellent appointments, highly qualified and experienced, and well grounded in science,” Rita Colwell, a microbiologist at the University of Maryland at College Park and a former director of the US National Science Foundation, wrote to Nature in an e-mail...
Tomorrow...tomorrow...tomorrow...it's only 14 hours away...
Some pictures from the process of making coffee.
I was catching up on my reading, and I came across this paper about coffee which claimed that coffee is the second largest consumer product after petroleum: Integrated Design of Biorefineries Based on Spent Coffee Grounds (Manuel Taifouris, Marcos L. Corazza, and Mariano Martín Industrial & Engineering Chemistry Research 2021 60 (1), 494-506).
This seemed like an extraordinary claim, so I decided to look at reference 5 in the paper, on which this claim was based. Reference 5 was this paper: Sustainable management of coffee industry by-products and value addition—A review
I'm not sure the claim is well supported in this paper (although I don't have time to read the entire paper now, but have downloaded for future reference.)
I'm a regular consumer of copious amounts of coffee and in looking at the pictures, I recognized that I have never thought in my life about what goes into the product and whence it comes.
Here's a few pictures of the coffee process beginning with the plant:

The caption:
The plant, which apparently originated in modern day Ethiopia is described like this:
Coffee leaves are opposite decussate on suckers. The leaves appear shiny, wavy, and dark green in color with conspicuous veins. The inflorescence is a condensed cymose type subtended by bracts. Coffee is a short day plant and hence the floral initiation takes place in short day conditions of 8–11 h of day light. Pollination takes place within 6 h after flowering (Fig. 2). Arabica coffee is autogamous with different degrees of natural cross-pollination in contrast to Robusta coffee, which is strictly allogamous with an inbuilt ametophytic system of self-compatibility. The process of fertilization is completed within 24–48 h after pollination. Seeds are elliptical or egg shaped and the seed coat is represented by the silver skin which is also made up of scleroides. The size, thickness or number of pits in the walls of scleroides is considered as important taxonomic characters in differentiating between species. Germination takes place in about 45 days.
Coffee in bloom:

The caption:
Coffee pulping:

The caption:
Coffee drying:

Coffee roasting:

The caption:
A diagram of the coffee "cherry" as obtained from the plant as a fruit:

A schematic of the coffee process with some byproducts:

The caption:
I think it's a good idea to appreciate whence our "stuff" comes.
Pretty cool, I think.
Trump's Financial Troubles May Be Just Beginning
An article in The New Yorker: Trump’s Financial Troubles May Be Just Beginning
Some excerpts:
It’s too early to determine whether Donald Trump might be headed for the same fate as the wretched Campbell, but there are intriguing parallels. Not so long ago, Trump also had lots of friends and creditors...
...His most important creditor was Deutsche Bank, which, during the past decade, had defied internal dissension and extended hundreds of millions of dollars of loans to him. On top of these invaluable relationships, Trump had nearly ninety million Twitter followers, a vast audience that appeared to provide lucrative possibilities for monetization after he left office.
But, in the week since Trump incited a mob of his supporters to attack the Capitol, he and his businesses have suffered a series of blows. Key corporate partners have abandoned him; some of his fellow-billionaires have spoken out against his sedition; Deutsche Bank has let it be known that it doesn’t want anything more to do with him; and Twitter stripped him of his following...
... Trump’s attempt at a Presidential “self-coup” came at what was already a troubled time for the Trump Organization, which has consistently struggled to eke out much in the way of taxable profits. As the coronavirus ravaged the travel and hospitality industries in 2020, Trump-owned hotels and golf resorts suffered along with other businesses in these sectors. Like other companies, the Trump Organization shuttered some of its properties for several months, including a hotel in Las Vegas and golf courses in Florida, Scotland, and Ireland. The virus also impacted two of Trump’s most valuable real-estate assets: a pair of prime office towers that he co-owns with Vornado Realty Trust...
...Shortly after Trump lost the election, according to the Washington Post, one of his closest business associates, the real-estate investor Tom Barrack, called Trump and advised him to abandon his efforts to overturn the result, and to opt, instead, for an “ ‘elegant’ exit...”
...Trump ignored it.
...After the deadly violence at the Capitol, on January 6th, the financial blowback on Trump came quickly. Within twenty-four hours, Shopify, the e-commerce provider, shut down a number of online stores affiliated with Trump, including ones that sold Trump campaign merchandise. A bigger blow for the President came last Sunday, when the P.G.A. of America, which has had a long-standing relationship with him, announced that the 2022 P.G.A. Championship, one of professional golf’s four major tournaments, would no longer be held at the Trump National Golf Club in Bedminster, New Jersey. Twenty-four hours later, the R. & A., the ruling body of golf outside of the United States, followed the P.G.A.’s lead and announced that, for the foreseeable future, the British Open would not be played at Turnberry, a famous old course that Trump owns in Scotland...
White House speech: "Big Data in Multiscale Modeling and Biology".
A Ph.D. student who I referenced in another post here, reported that she was inspired by Dr. Ivet Bahar.
I didn't know who she was, so I googled my way to her Wikipedia page.
Ivet Bahar
Can you imagine the philistine still in the White House, and soon to be unceremoniously escorted out of it, inviting someone to give that lecture?
It will be so good to have an intelligent and decent human being in the White House again.
Now that the racist thug has become a pariah to whom little attention is being paid, it's great to be seeing our President Elect transition to office. I want to weep for joy.
The next person that searches for Black women in computational biology...
There's a new scientific journal (like we needed more?) called Nature Computational Science .
For the inaugural issue which happens to be published inaugural week when decency is restored to the US Presidency, there is this wonderful article about the future we all desire, here at least: Connecting Black women in computational biology (Chirigati, F., Rastogi, A. Connecting Black women in computational biology. Nat Comput Sci 1, 11–13 (2021). )
It should be open sourced, but some excerpts from the interview with Jenea Adams, a second year PhD student who created the Black Women in Computational Biology Network:
...My main role model was Ivet Bahar from the University of Pittsburgh, who was my advisor during my REU program. I saw a really powerful woman leading an important department at the university and in computational science, and she inspired me to reach for more and think outside of my comfort zone. At the time, I didn’t know many women in the field, and I certainly didn’t know any women who looked like me in the field, so she was definitely inspiring to me...
... It was honestly a natural process. I decided to search for ‘number of Black women with degrees in computational biology’ on the Internet and found nothing. There was an editorial on women in the field, but nothing relevant to race or ethnicity demographics of the field. I didn’t put that much thought into it, but I knew that someone like me was going to really appreciate being able to find other Black women by doing a simple search...
... Now, we have a new website that is so much more and symbolizes our growth into a full organization. The next person that searches for Black women in computational biology will be able to find us...
I have no comment to add except kudos to Ms. (eventually "Dr." ) Adams
Exploration of the Plutonium/Plutonium Hydride Phase Diagram.
(Graphics in this and previous posts may not be visible in Google Chrome, but should show up in Microsoft Edge, Firefox and Android.)
The paper I'll discuss in this post is this one: Quantum Accurate Prediction of Plutonium–Plutonium Dihydride Phase Equilibrium Using a Lattice Gas Model (Ryan Gotchy Mullen and Nir Goldman, The Journal of Physical Chemistry C 2020 124 (38), 20881-20888).
Not so long ago in this space, I noted that in nuclear fission, some americium isotopes exhibit a very high yield of neutrons, called generally "multiplicity," and thus are capable of extraordinary breeding ratios: Electrochemical oxidation of 243Am(III) in nitric acid by a terpyridyl-derivatized electrode.
Although americium will always be produced in a fission nuclear reactor having any kind of fuel efficient high burn up, the reality is that, even though Am-241, the lightest commonly available americium isotope, exhibits high multiplicity in the fast neutron spectrum, it is still 3 mass units separated from its natural source, uranium-238. It is also true that there is not enough americium on the planet - even though inventories in used nuclear fuel are significant - to work toward the immediate elimination of dangerous fossil fuels while still preserving human development goals and the elimination of poverty.
There is, by contrast, enough plutonium available in used nuclear fuel to power all of the world's energy demand, roughly 600 EJ/year, by utilizing a type of nuclear reactor currently under commercial development by a number of companies and laboratories: The "Breed and Burn" type reactor, Sekimoto's "Candle Reactor" and variants thereof. This strategy would be sufficient to provide all of humanity's energy needs for centuries without operating a single energy mine of any kind anywhere on the planet using uranium already mined and isolated - including "depleted uranium" - as well as the thorium mined and dumped in order to serve the ridiculous so called "renewable" wind industry and other lanthanide dependent industries.
Light A Candle, An Innovative Burnup Strategy for Nuclear Reactors.
In order to assure that sufficient plutonium remains available for this purpose, as well as to provide for other uses for neutrons beyond fuel breeding, it is desirable to have as high a multiplicity as possible for plutonium. In the fast neutron spectrum, plutonium is always a breeder fuel, but how well it breeds is a function of the chemical form of the element, for example, whether it exist as oxides or nitrides or some other compound. Metallic plutonium, although it has a complex phase diagram, gives the highest multiplicity among these options. Very old literature, from the 1960's, indicates that the highest multiplicity ever observed in plutonium, giving a breeding ratio of over 1.5, is available in liquid plutonium. (cf. E.V. Evans, Editor, Fast Breeder Reactors, Proceedings of the London Conference on Fast Breeder Reactors, 17-19 May, 1966, Whitman, in "Fast Breeder Reactor Development in the United States, paper 3/2 pg. 286 )
I have been thinking about and studying the literature associated with liquid plutonium for a number of years now with particular attention to an experimental reactor that was being described in the 1966 reference just above, which operated at Los Alamos nearly sixty years ago, the LAMPRE reactor. This reactor utilized tantalum capsules to contain the plutonium, tantalum and tungsten being the only two metals that do not dissolve in liquid plutonium. Tantalum was selected because of the difficulty of machining and welding tungsten.
No device dependent on tantalum can be considered sustainable, since the element is a "critical element," one which is easily subject to depletion with rising use. In addition, it is a "conflict element," an element mined under appalling conditions often involving human slavery, where some of the slaves are children. (The main use for the element today is for supercapacitors in cell phones.)
It does seem to me that advances in materials science over the last 60 years may well eliminate the need for tantalum for the purpose for liquid plutonium, and that composite ceramics may be the key to solving this technical problem.
During operations of LAMPRE, it was noted that the surface of the liquid plutonium during operation became coated with a solid material. To my knowledge it was not chemically analyzed, but most likely it was metallic and contained fission products like the element strontium (which is insoluble in liquid plutonium and has a melting point of 777°C, higher than the LAMPRE operating temperature) as well as intermetallic phases like those between zirconium, a fission product, and plutonium.
(Plutonium Handbook, O.J. Wick Ed., M.D. Freshly, Vol 2, Chap 20 . pg 662-664, Gordon and Breach Scientific Publishers 1967. This offers a fairly illuminating, if brief, description of the irradiation of a liquid plutonium/iron eutectic in the LAMPRE reactor.)
Here is the phase binary diagram of the zirconium-plutonium system:
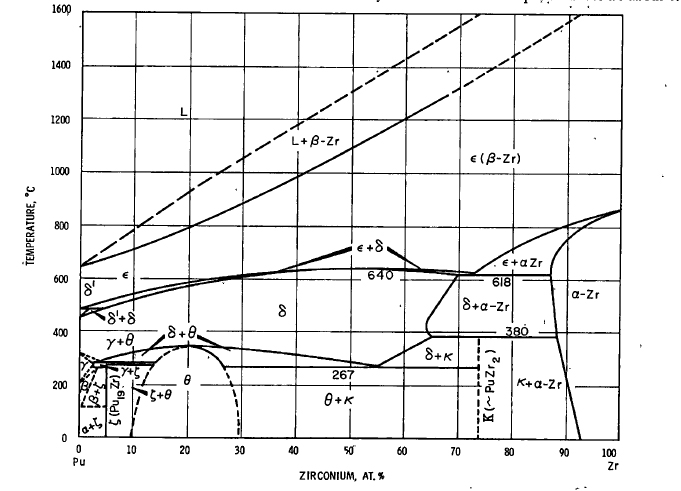
(cf. Plutonium Handbook, O.J. Wick Ed. Ellinger, Land and Gschneidner, Vol 1, Chap7. pg 227, Gordon and Breach Scientific Publishers 1967)
Although this binary phase diagram comes from a reference now 53 years old, it is essentially equivalent to the ATSM database version published in 2007 which includes more recent references, with the benefit that this older version is better labeled with allotropes as opposed to more arcane space groups accessed by keys in the ASM version. It is notable that the ASM version shows more completely the closing of the ellipsoid Zr solid + Pu liquid region at increasing Zr concentrations up to the melting point of zirconium, 1855°C, as one should well expect.
An aptly named review article from last year refers to this phase diagram as well as CALPHAD (Computer Coupling of Phase Diagrams and Thermochemistry) models and other updates: Experimental and Modeling Review of the Plutonium-Zirconium (Pu-Zr) System: Lost in Translation and Over Time? (Aurélien Perron & Patrice E. A. Turchi , ( J. Phase Equilib. Diffus. 41, 756–763 (2020))
In any case, this binary diagram is a vast over simplification of the putative nature of the solid phase on the LAMPRE fuel surface, but does indicate that it is possible to saturate liquid plutonium with zirconium. I have in my files, more complex ternary phase diagrams, for example, the iron zirconium plutonium system and the iron uranium zirconium system, but I will spare the reader these. Real systems are even more complex, given the plethora of elements in the periodic table present as fission products and/or structural materials.
Nevertheless, these complex systems suggest the possibility of peritectoid systems, in which solid phases crystallize out of solutions. It is a property of peritectics that on occasion they can represent inert coatings, since they are quasi-stable at the peritectoid point, with limitations in further penetrations of a given element into this system, something that was not likely explored very deeply half a century ago.
Although historically it was believed that compound formation was not involved in these phases, it seems that the modern interpretation is quite different and that several of the phases are indeed thought to be peritectoid in nature.

References 4 and 15, however, date from the 1960's.
Nevertheless one can look at something approximating a "synthetic peritectoid" of plutonium to moderate the rate of corrosion in the design of a nuclear reactor that is in fact, designed to eat through its fuel. Modern computational capabilities, not available in the period between 1960-1980 -when the bulk of our nuclear fleet much of which still operates today was designed and built - can certainly play a role in materials design that must be employed to allow nuclear energy to do what it must do to save the world from the ongoing disaster of climate change.
Thus examination of the phase diagrams of plutonium with other elements always catch my eye. Hydrogen is moderating, of course, which precludes a "breed and burn" system, so it's not clear that this particular system would be useful as a containment strategy exploiting the benefits of liquid plutonium. But it is the process, and not the result, which is most important here.
From the introduction to the text:
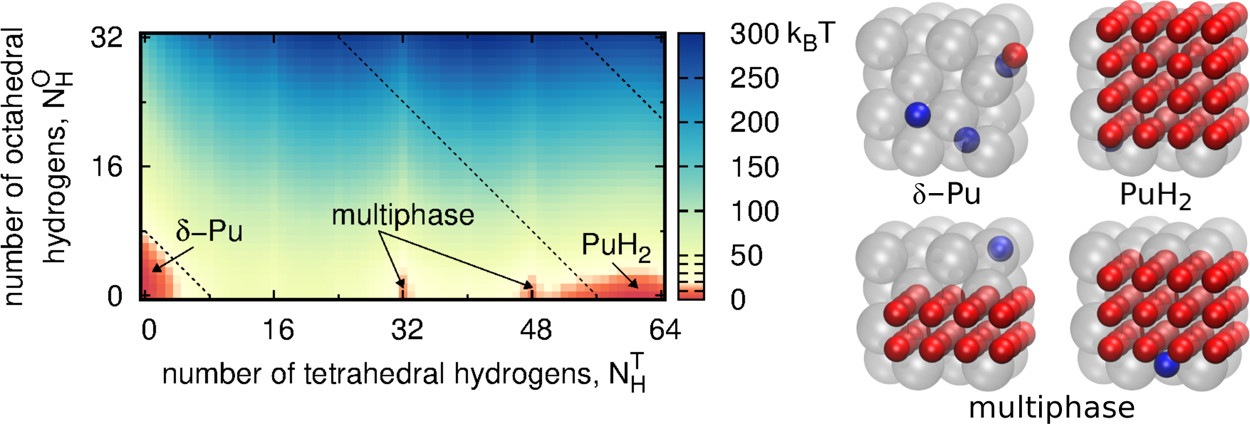
The complexities of plutonium hydriding can be illustrated by a comparison to hydriding in a more common metal, such as palladium. The critical temperature of the palladium–palladium hydride phase envelope is 550 K (277 °C), well below the palladium metal melting temperature of 1828 K (1555 °C).(4) In contrast, solid plutonium dihydride (PuH2) will crystallize from liquid Pu that is exposed to hydrogen gas.(5) In addition, hydriding in face-centered cubic (fcc) ?-Pu (of interest for engineering applications because of its high ductility(6)) induces a large volume expansion of 54%, compared to an expansion of only ?10% in palladium hydriding, resulting in flaking and the complete degradation of Pu-based materials because of this lattice mismatch. Finally, neutron diffraction reveals that H atoms randomly occupy interstitial octahedral (O) sites in palladium hydride.(7) In contrast, no neutron diffraction studies have been performed on ?-Pu or PuH2 to indicate how H atoms partition between O sites and tetrahedral (T) sites, though investigations of lanthanide hydrides show that O sites are not occupied until all available T sites are filled.(8)
Conducting experiments with plutonium is exceedingly difficult because of its toxicity and radioactivity. The lowest temperature at which the composition of the PuH2 phase has been reported is 773 K (500 °C).(5) Mulford and Sturdy additionally reported equilibrium pressures at 673 and 723 K (400 and 450 °C) but were unable to determine the equilibrium compositions at these temperatures, reporting that it took 20 h to equilibrate at each trial composition...
I have always known that plutonium is a very active metal, but the idea that hydrides are involved in the dissociative absorption of water into the metal is something I had not considered. This should have some implications in connection with the long term stability of nuclear weapons, reducing their reliability - a good thing - I would think if the core is not desiccated with regular maintenance to address saturation of the desiccant would further reduce their viability of weapons. (We need to do a "sword into ploughshares" kind of thing with plutonium, something with which VP Al Gore had notable success with negotiating with Russia in the 1990's with enriched uranium, pre-Putin of course.)
Later on, the introduction gives the paper's raison d’être:
Ga-stabilized ?-Pu is the form of plutonium utilized in nuclear weapons. An interesting and fun bit of history is that the Russians and the Americans each figured out in the 1950's that they both were using this alloy in their weapons because the gallium/plutonium alloy was the only phase diagram that was consistently not published in the open scientific literature because it was regarded as "classified."
The paper goes on to discuss the issue of computer time required to do various types of calculations associated with computer models, density function theory (DFT) for example and lattice gas models. On a simplistic level, these calculations should be expected to be computationally expensive given the fact that plutonium metal involves 94 electrons for each atom, but great strides have been made in the last 50 years in accessing meaningful data using approximations, a task that was initiated, among other places, with the Born-Oppenheimer approximation which assumed static nuclei. People with a college level background in quantum physics/chemistry will recall that exact solutions to energy calculations only exist for the (unbonded) hydrogen atom, and all other calculations in all other systems require iterative calculations that converge on solutions. These systems have become increasingly sophisticated and useful. Just one example is Orbital-free density functional theory which is an "electron gas" model for finding the solutions which the Kohn-Sham theorem predicts.
In the text a simplification utilized is to restrict hydrogen atoms in plutonium hydride to tetrahedral (T) and octahedral (O) sites in the lattice.
Some sample math porn from the paper:
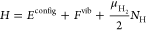
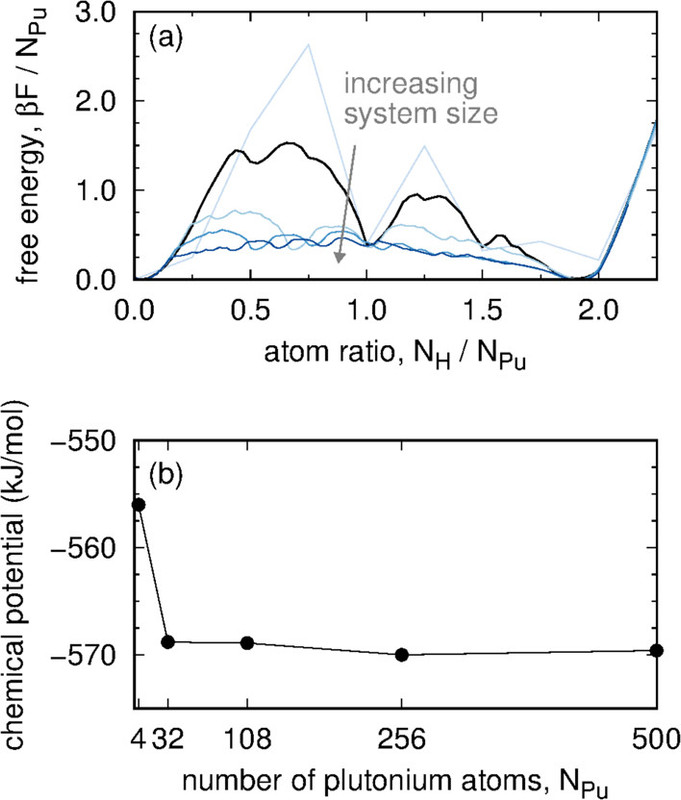
where the configurational potential energy Econfig, the vibrational free energy Fvib, and number of hydrogen atoms NH are properties of the microstate while the chemical potential of a gas-phase hydrogen molecule ?H2, the number of plutonium atoms NPu, and the temperature T are held constant. Each absorbed H is treated as an independent quantum harmonic oscillator. Consequently, eq 1 contains the analytic free energy Fvib rather than a potential energy Evib and corresponding quantum degrees of freedom. A P?V term for H in the Pu lattice is not included in eq 1 because under the conditions studied here, its value is several orders of magnitude smaller than other terms. The impact of plutonium defects on Pu–PuH2 equilibrium is not considered in this work...
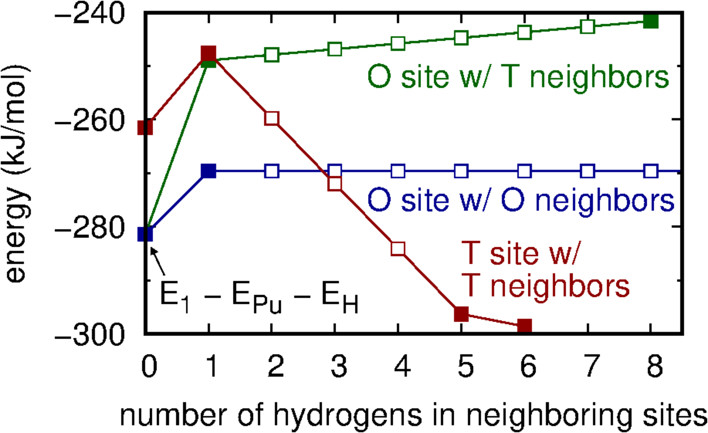
...Because of the likely strong screening effects of the intervening plutonium atoms, we parameterize the change in energy using only the number of occupied nearest neighbor sites surrounding an occupied O or T site. This results in three types of H–H structures: those comprised of hydrogens in neighboring T sites (ETT), those in neighboring O sites (EOO), and cross-interactions (EOT). As will be shown, all OO and OT structures are sufficiently energetically prohibitive as to be rare in either ?-Pu or PuH2 phases. Accordingly, we ignore the change in energy induced by an OO structure when OT structures are present because the latter increase the energy significantly more than the former. The configurational potential energy is
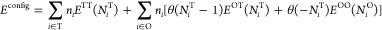
where ni is 1 if site i is occupied and 0 otherwise, and the function EOT accounts for H–H interactions between a hydrogen in an O site and the NiT hydrogens in the nearest neighbor T sites. ETT, EOO, and NiO are defined similarly. The number of occupied neighbors NiT and NiO are computed using periodic boundary conditions. The first sum is over T sites, the second sum is over O sites, and the Heaviside function ? (x) is 1 if x ? 0 and 0 otherwise.
Zero-point energies and entropic contributions from the vibration of light interstitials such as H in metals can be significant. Changes in the plutonium vibrational frequencies are not included because Pu atoms are much heavier than the absorbed H atoms. The insertion of a hydrogen atom, on the other hand, creates three vibrational modes that did not exist in the previous configuration. These modes are treated as quantum harmonic oscillators, with the following analytical free-energy expression
where ni is 1 if site i is occupied and 0 otherwise, and the function EOT accounts for H–H interactions between a hydrogen in an O site and the NiT hydrogens in the nearest neighbor T sites. ETT, EOO, and NiO are defined similarly. The number of occupied neighbors NiT and NiO are computed using periodic boundary conditions. The first sum is over T sites, the second sum is over O sites, and the Heaviside function ? (x) is 1 if x ? 0 and 0 otherwise.
Zero-point energies and entropic contributions from the vibration of light interstitials such as H in metals can be significant. Changes in the plutonium vibrational frequencies are not included because Pu atoms are much heavier than the absorbed H atoms. The insertion of a hydrogen atom, on the other hand, creates three vibrational modes that did not exist in the previous configuration. These modes are treated as quantum harmonic oscillators, with the following analytical free-energy expression
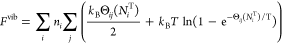
Here, ?ij is the vibrational temperature ℏ?ij/kB for frequency ?ij of a hydrogen in site i along normal mode j. We parameterized ?ij as a function of NiT to account for the decrease in ?ij as the plutonium lattice expands with the absorption of hydrogen...
Some pictures from the text:
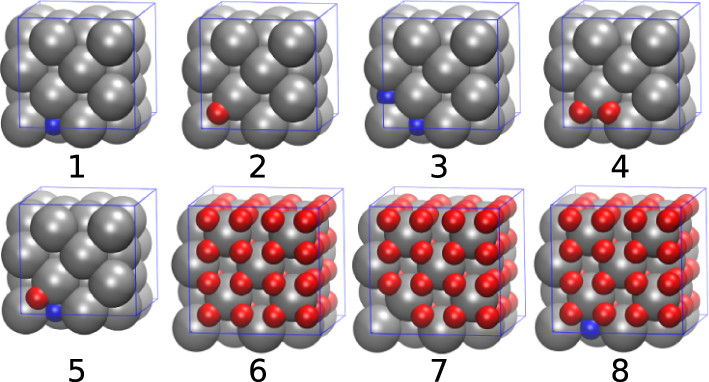
The caption:
The caption:
The caption:
The caption:
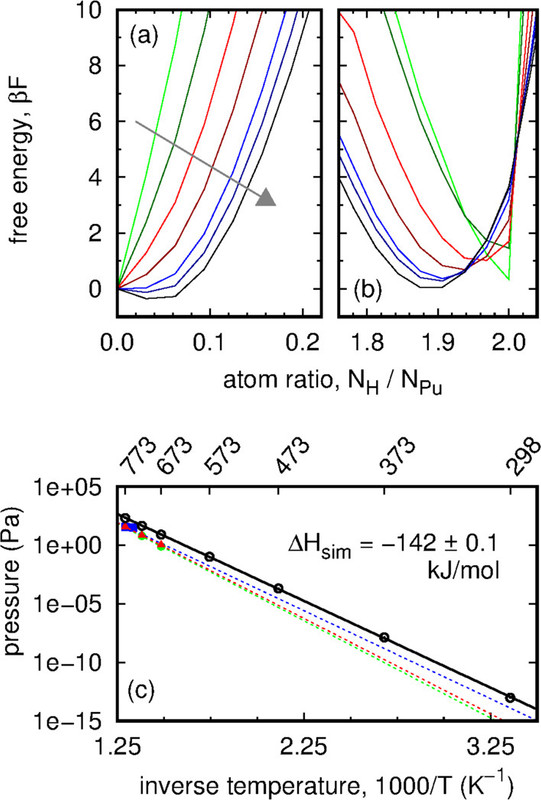
The caption:
From the conclusion to the paper:
...We anticipate that our lattice gas model could be parameterized to examine the hydride phase envelopes of other fcc actinides, rare earth metals, or transition metals (e.g., thorium, cerium, and palladium). Overall, our predictions provide a baseline to guide future plutonium experiments...
I'm not shy in offering my opinion that if we are to save the world, we should definitely have lots and lots of "future plutonium experiments"
I trust you're having a nice and safe weekend, as we all look forward to having a real President of the United States again in this coming week.
Large Scale Continuous Flow Synthesis En Route to the Synthesis of the Antiviral Remsdesivir.
The paper to which I'll briefly refer (it's open sourced) to make a point relevant to our current situation is this one: Development of a Large-Scale Cyanation Process Using Continuous Flow Chemistry En Route to the Synthesis of Remdesivir (Tiago Vieira, Andrew C. Stevens, Andrei Chtchemelinine, Detian Gao, Pavel Badalov, and Lars Heumann Organic Process Research & Development 2020 24 (10), 2113-2121)
Today, between normal duties, I took a little time out to attend some lectures put together by my section of the ACS and hosted by Professor Spencer Knapp of Rutgers, on Zoom: 2021 Synthesis on Scale Symposium One of the speakers was Lars Heumann of Gilead, who described beautifully the total synthesis of Remdesivir.
I know...I know...I know...
I'm used to hearing all the time about how "evil" Gilead is because their making a ton of money on the antiviral remdesivir which is a controversial and marginally effective drug in the treatment of Covid-19.
I know...I know...I know...
Donald Rumsfeld had a position in Gilead.
The pharmaceutical industry is evil...I know...I know...I know...
I guess it would be too much to appreciate all the hard work that goes into producing a drug after its discovered. The task of the medicinal chemist is to simply make molecules by the most effective quick route for the purpose of screening. It is rare that the syntheses in this context can be economically or viably scaled up to production levels.
Although I'm no longer actively as engaged as I once was in process chemistry support, I remember very well the period in which the scale up of HIV drugs was at the forefront of the industry. I was a foot soldier in a veritable army of scientists who solved incredible problems, coordinating logistics from all over the world, quite literally, to bring those drugs to market.
Dr. Heumann's lecture reminded be of those days.
Remsdesivir was developed for the treatment of Ebola, where it is clearly effective. It was done in an extremely high pressure environment, with the synthesis going from milligrams to kilograms (to support early clinical trials) in a matter of months. Making the drug on a ton scale was also required in a matter of months.
The drug is a nucleoside mimetic which interferes with viral reverse transcription from viral RNA to active DNA utilized for the synthesis of viral particles.
Here is a total synthesis of remdesivir, a variant of which, on scale, Dr. Heumann walked us through today:

It is described, also open sourced here: Discovery and Synthesis of a Phosphoramidate Prodrug of a Pyrrolo[2,1-f[triazin-4-amino] Adenine C-Nucleoside (GS-5734) for the Treatment of Ebola and Emerging Viruses] (Dustin Siegel, Hon C. Hui, Edward Doerffler, Michael O. Clarke, Kwon Chun, Lijun Zhang, Sean Neville, Ernest Carra, Willard Lew, Bruce Ross, Queenie Wang, Lydia Wolfe, Robert Jordan, Veronica Soloveva, John Knox, Jason Perry, Michel Perron, Kirsten M. Stray, Ona Barauskas, Joy Y. Feng, Yili Xu, Gary Lee, Arnold L. Rheingold, Adrian S. Ray, Roy Bannister, Robert Strickley, Swami Swaminathan, William A. Lee, Sina Bavari, Tomas Cihlar, Michael K. Lo, Travis K. Warren, and Richard L. Mackman
Journal of Medicinal Chemistry 2017 60 (5), 1648-1661)
The length of the list of authors should give some insight to how much work went into this project. When one considers all of the training that went into the education of this authors, one's mind should be blown.
I know...I know...I know...Donald Rumsfeld...profits....gasp.
The final industrial synthesis, is similar.
What is beautiful about the synthesis, as is described in the paper linked at the outset is that this process is a continuous flow process, as opposed to a batch process.
Continuous flow processes are cleaner, safer, more reliable, and generally cheaper than batch processes. Since there was a risk of generating hydrogen cyanide gas in the reaction, this was a big deal. Dr. Heumann's lecture featured some photographs of chemists and chemical operators in full protective suits with oxygen tanks and handy cyanide antidotes, during one step in the synthesis.
These people quite literally risked their lives to make this drug.
That matters.
I don't think people can really grasp that, understand that. They think that drugs come out of a magic faucet somehow, I think.
I cannot tell you what goes into scale up of drugs and vaccines in a brief post, but it does involve incredible sacrifice, and we should appreciate that on some level it can be heroic.
Here is a photograph, from a recent news section of Nature of reactors in which the Covid-Vaccines are being prepared:

How COVID unlocked the power of RNA vaccines (Elie Dolgin, Nature News January 12, 2021.)
Have a nice weekend.
Science on Saturday lecture: How to Recognize AI Snake Oil.
PPPL's science on Saturday lecture tomorrow morning by Princeton University Professor Arvind Narayanan is entitled "How to Recognize AI Snake Oil."
Sign up here:
Science on Saturday, on Zoom
Dr. Narayanan spoke a few years back when Science on Saturday was being held live at PPPL. I recall it as being quite interesting. (It's very, very, very rare when a PPPL Science on Saturday is not interesting.)
As the talks have moved to Zoom, they are now accessible around the world. They are held at 9:30 am EST, with a Q&A session after the talks ending usually by 11:30. The talks themselves are about an hour generally.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,598