NNadir
NNadir's JournalRecovery of Trivalent Lanthanides and Transplutonium Actinides with Resin Supported Diglycomides.
The paper I'll discuss in this post is this one: Scaling Trivalent Actinide and Lanthanide Recovery by Diglycolamide Resin from Savannah River Site’s Mark-18A Targets (Kevin P. McCann, Mark A. Jones, Edward A. Kyser, Tara E. Smith, and Nicholas J. Bridges Industrial & Engineering Chemistry Research 2021 60 (1), 507-513).
The elements in the two rows below the "main" periodic table are collectively called the "f elements." The row beginning with the chemical symbol Ln (lanthanum) are called "the lanthanides" - and somewhat more commonly in an annoying and misleading term, "rare earths" - and the row beginning with the chemical symbol Ac (actinium) are called the "actinides." They are placed below the main elements of the periodic table only to make the table fit nicely in the width of a sheet of paper. Properly drawn they represent another "step" in the step shapes in the main part of the table, properly the table should have 32 columns, not 18, although the congener relationship in purely chemical terms between, say protactinium and praseodymium is weak and not all that worthy of consideration.
The lanthanides, with some exceptions, generally exhibit the +3 oxidation state, the "trivalent" state, because the "f orbitals" which are being filled across the row do not participate to any appreciable extent in chemical bonding because of shielding effects.
This is not true for the lower actinides before americium, only actinium itself exhibits only the trivalent state. For a very long time, until the 1940's, when interest in actinide chemistry exploded - no pun intended - thorium was thought of as a congener of hafnium and zirconium, because like them, its most common oxidation state is +4, and protactinium was considered a congener niobium and tantalum because its common oxidation state is +5, and uranium, a congener of molybdenum and tungsten because of its common oxidation state of +6.
Actinium, thorium, protactinium, and uranium all occur naturally in weighable amounts, thorium and uranium on a billion ton scale - their decay is largely responsible for the internal heat of the Earth - actinium and protactinium occur in trace amounts, in concentrations so low that they are best accessed not by isolation from the ores in which they occur, but by the use of nuclear reactions, neutron or proton bombardment.
In 1940's, as he worked on the discovery of new synthetic elements in the periodic table, especially neptunium, plutonium, americium and curium, Glenn Seaborg had the insight that the chemistry of these elements could be discerned by recognizing that they were, in fact, "f elements" as opposed to "d elements" like hafnium, tantalum, and tungsten.
The actinides become "lanthanide-like" at americium. Although americium can be oxidized to higher oxidation states, it's major oxidation state is +3; this is also the case with curium, berkelium and californium.
When I was a kid, the first mass spec with which I was used by one of the companies for which I worked had a californium ionization source; I'm an old guy. (Modern mass specs have other types of ionization inductions, notably electrospray ionization (ESI) for which John Fenn won the Nobel Prize.) The californium where I worked (in California) was the 252 isotope, which has a half-life of about 2.64 years, decaying both by spontaneous fission and by alpha decay to curium-248. The spontaneous fission of californium-252 made it a useful source of neutrons, and it was widely used in chemical analysis using neutron activation analysis, which has been mostly displace by high sensitivity ICP/MS instruments that for most elements can record parts per trillion.
Perhaps a current motivation, particularly in the days of antibody payloads is in neutron boron therapy, where small portable Cf-252 sources might displace the need for expensive accelerators, particular in rural or remote regions: Boron neutron capture therapy: Current status and future perspectives (Dymova, M.A., Taskaev, S.Y., Richter, V.A. and Kuligina, E.V. (2020), Cancer Commun., 40: i-i.)
In the 1960s and 1970's an effort was made to produce large (large being milligram quantities) of californium-252 as a neutron source and as an ionization source. (Mass specs in space robots generally use curium sources because of their longer half-lives.)
Another important isotope is plutonium-244, which has a half-life of 80 million years, and which is used as an internal standard in actinide analysis, and as a target for super-heavy element analysis.
The United States is running out of plutonium-244, and the paper listed above is about recovering it, as well as the transplutonium elements therein. The introduction to the paper covers things on which I touched above:
To recover the valuable materials, the Mark-18A material recovery flowsheet separates the target’s aluminum cladding by caustic dissolution, leaving most of the fission products and the actinides as a solid material. The undissolved material is filtered and then subsequently dissolved in >7 M HNO3 at elevated temperatures. The resulting high nitrate solution, containing dissolved plutonium, actinides, and most of the fission products, will be sent through an anion exchange column using Reillex HPQ resin, similar to its use in the SRS’s HB-Line Facility.(10?13) Reillex HPQ achieves Pu/Am decontamination factors on the order of 14,000.(11,12) The high decontamination factors are a result of Reillex HPQ’s high selectivity for the Pu(NO3)6 2– anion at >7 M HNO3 and little to no affinity for lanthanides (Ln), americium, curium, and fission products. As a result, in the Mark-18A material recovery flowsheet, the Ln, Am, Cm, and remaining fission products remain in the raffinate and require additional processing to recover heavy Cm from the >7 M HNO3 raffinate...
The Reillex HPQ resin is an anion exchange resin, a polyvinyl-N-methylpyridinium resin, that intereacts with the anionic plutonium (IV) hexanitrate anion (-2). In Reillex 402, the methyl group is replaced by a proton, in other N-pyridinium alkyl primary ammonium groups of various chain lengths to give a dicationic species.
The structure of these complexes are nicely shown in this cartoon:

Molecularly Engineered Resins for Plutonium Recovery
(S. Fredric Marsh, D. Kirk Veirs, Gordon D. Jarvinen, Mary E. Barr, and Eddie W. Moody, Los Alamos Science 26 (2000) 454-463)
The Los Alamos people note that some of the designed resins may also extract transplutonium nitrate complexes, but it would appearl that straight up Reillex HPQ (the methyl pyridinium is selective toward tetravalent plutonium nitrate complexes) and thus can be utilized in the current setting, where the target are trivalent species, specifically transplutonium actinides and lanthanides.
To save the world, the required inventory of plutonium - even in the "breed and burn" scenario where only a critical mass (realistically a little more) is required for start up - is on the order of hundreds of metric tons, and it is unrealistic that Reillex HPQ would be of much use on that scale.
These charged resins thrill, perhaps in the form of ionic liquids, the imagination about the possibility of electroprocessing.
I recall that when I was writing over in the E&E forum a lot, an anti-nuke of the particularly dull sort announced that nuclear energy was "too dangerous" because a tunnel at the Hanford site which had been abandoned collapsed. Pretty typically this person who is happily on my ignore list was far less interested in the 7 million people who died last year from air pollution while dumb guys get wedgies in their underwear about tunnels built in the 1950's which may contain (gasp) radioactivity. On investigation, it turns out that the tunnel contained some old abandoned chemical reactor vessels for plutonium purification ob rail cars, with trace plutonium on their surfaces. The number of lives lost associated with the collapsed rail tunnel was zero.
Reillex HPQ would be good to decontaminate the decontamination wash solutions to clean off the chemical reactors such as may exist from reprocessing efforts, but not for large scale processing. Let's be clear though, on a scale of risk, when compared with the very real and rising catastrophe of dangerous fossil fuels, unless one is a complete idiot - and sadly complete idiots exist - plutonium stains on a 50 year old chemical vessel is a non starter.
But in the case of the Mark-18A targets, these contain curium on a scale of a few hundred grams, and thus the resins have much to recommend them. Since the quantities are relatively small, but extremely valuable, column scale separations are entirely acceptable.
The authors note that during the last campaign to milk the Mark-18A targets for their valuable components, which took place in the early 1970s, a Dowex anion exchange resin was utilized. A difficulty with this resin, although it was clearly workable, was the release of sulfate from the sulfonylphenyl groups on which this relatively primitive anion exchange resin was based. Sulfates tended to contaminate the eluted products and raffinates, requiring additional clean up steps. Moreover, combustion of the used resins was problematic.
The more modern resin under discussion here contains only carbon oxygen and thus can be readily destroyed in an appropriate oxidation setting designed to contain residues.
The historical separation process is described as follows:
To prevent leaks, the authors were looking for a way to avoid the use of pressure, hence the evaluation of a new approach to the separations. All of the fermium and einsteinium in the targets has now decayed to lower actinides, of course, so they are no longer relevant to the case.
They write:
Although I do not have access to the exact structure of the commercial DGA resin, the structure of these resins is probably something along these lines:

(cf Mohapatra et al., RSC Adv., 2014,4, 10412-10419)
The authors conducted two tests, one using non radioactive fission product simulant with neodymium standing in for the actinides, and a second, also loaded with a fission product simulant spiked with the actinide americium.
The flow chart calls for the dissolution of the aluminum clad targets with caustic (which dissolves aluminum), leaving behind a solid residue of oxides which are then dissolved in 7M nitric acid. It is the nitrate complex which is separated as anionic species by the resin.
Some pictures from the text:

The caption:

The caption:

The caption:
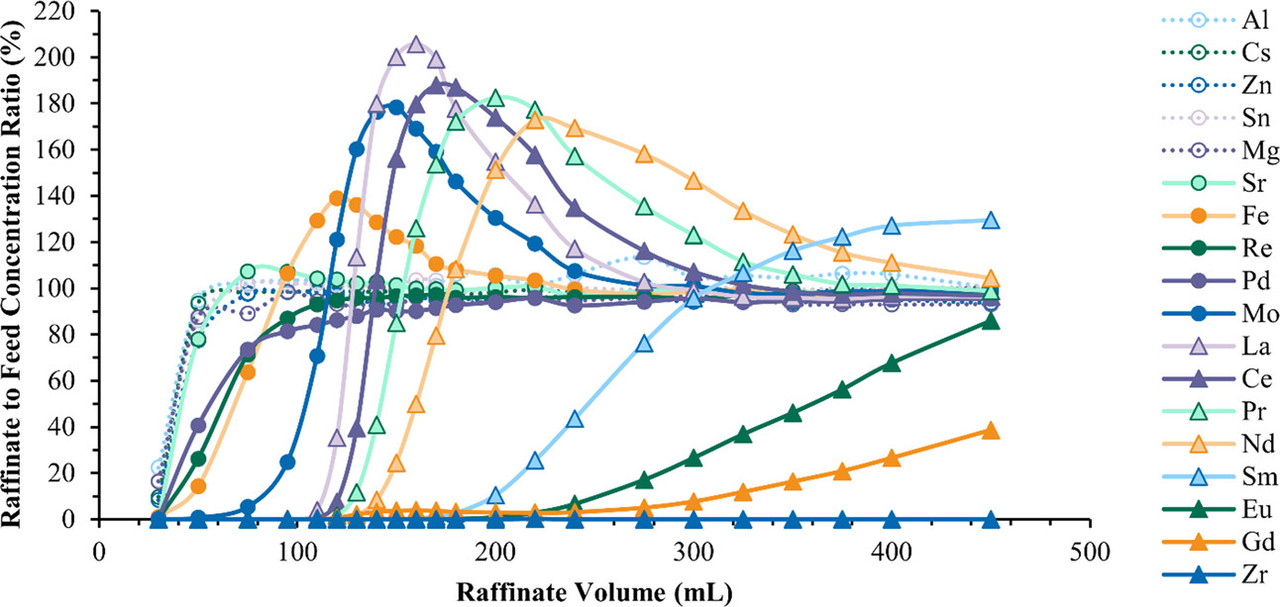
The caption:

The caption:
A new wrinkle in this method as opposed to the method utilized in the 1970s also concerns the detection. For the purposes of these experiments, the complexes were monitored by their UV absorption spectra.
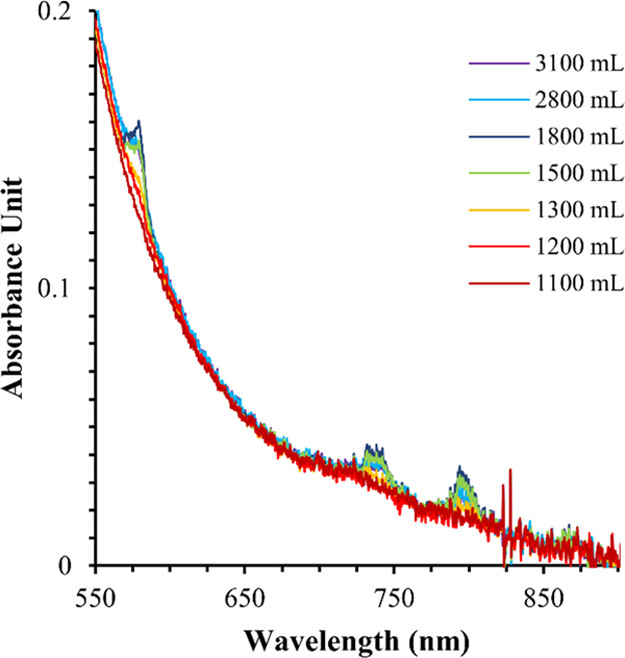
The caption:

The caption:
The authors conclude as follows:
...Americium tracer experiments validated that Am breakthrough closely follows Nd breakthrough. An in-line UV/vis spectrophotometer was able to track the ingrowth of Nd in the raffinate solution. In full-scale processes, detection of Nd in the in-line UV/vis system will indicate Am breakthrough and serve as a warning that Cm breakthrough is forthcoming. Future work will focus on column design for the full-scale process in SRNL hot cells. Additionally, research is underway to apply the CHON principle and incinerate the loaded DGA Resin to reduce volume while directly converting the loaded metal to a stable oxide form for shipment.
Cool paper I think, with some application, for cleaning chemical reaction vessels used in the essential - if we are to save the world - separation of the higher actinides.
I trust you are enjoying, as much as I am, the first weekend in the already magnificent Presidency of Joe Biden, and are doing so safely.
Conspiracy (2001), Kritzinger's Warning.
The Wannseekonference took place on January 20, 1942. The conference lasted about 2 hours, and was conducted as a lunch meeting. It was held to plan the murder of the Jews of Nazi occupied Europe, and discussed plans for the murder of Jews in countries that had not yet been conquered by Germany, Great Britain, for instance.
The participants were highly educated and efficient men.
The conference was organized by Reinhard Heydrich, a talented musician, skilled athlete who was educated at a German Naval Institute. He later became the "protector" of Moravia and Bohemia, where he was assassinated by British trained Czech partisans several months after the Wannsee conference. Heydrich is considered by historians to have been one of the purest embodiments of pure Nazi evil.
Notes from the meeting were discovered in 1947 from the files of Martin Luther, a minor Nazi official, probably the lowest ranking figure at the meeting, who had, in fact, ended up in a concentration camp himself before the war was over.
Several films, including documentaries have been made about the Wannsee conference, one in German in 1984, another in English in 2001, called "Conspiracy." It stars Kenneth Branagh in a chilling portrayal of Heydrich.
If one has ever been to a high level executive meeting in any capacity, one can recognize the tenor of the meeting as portrayed in "Conspiracy." The goal is to "get things done" while schmoozing in a genial way, juggling for position, clarifying issues, coming to a final agreement and understanding.
In this sense it is frightening, and I advise young people "on their way up" in their careers to study it in this light, to see how easy it can be to slip into a kind of officiousness that is divorced in every way from morality, how easy it is to divorce one's self from ethics by "going along," "not making waves." There is surely an element of that in all kinds of business meetings, including those where the business is criminal.
A scene in the film came to mind, because I have felt myself being consumed with rage and anger and, yes, frankly hatred over the last four years.
The scene comes at the end of the film, after all of the participants in the conference have left, after agreeing in an affable business-like sense to murder all of Europe's Jews in a two hour meeting. Heydrich (Branagh), Adolf Eichmann (played by Stanley Tucci), and Heinrich Mueller, head of the Gestapo, sit together to have a drink and commiserate about the outcome of the meeting. They discuss a story told by Friedrich Wilhelm Kritzinger, a State Secretary in the chancellery, who is portrayed in the film as having had reservations about the murder of the Jews, although it is not clear in the historical record that this actually was the case for the real Kritzinger.
The scene has stuck in my mind for many years as a kind of warning should one let oneself be consumed and defined by hatred.
No one here, I'm convinced, is consumed by race based hatred like Heydrich's Eichmann's and Mueller's hatred of the Jews, but I know for myself, I have repeatedly felt consumed by perhaps a more justified hatred over the last 4 years, which, irrespective of its origins and its justifications, is hatred all the same.
Speaking only for myself, in these days of liberation and relief, I want to let go of it; as hard as it is, I want to let go of the hatred. It does nothing good for me.
Still high...
Amanda Gorman's poem deepens my conviction that this coming generation is going to a great generation like none we've seen, and Yo Yo Ma, the beautiful comforting voice, the happiness, the Amazing Grace.
And then there's that old white guy, sprinting around the White House filled with fire, as if with a magic wand, expelling demons.
That old white guy makes me feel so much better about being an old white guy, which has been something of an embarrassment of late.
Suddenly I feel, at least, the joy of youth.
Yo Yo Ma, how beautiful, how unbelievably beautiful:
This old man is glad to have lived to see this!!!!!
What is the status of the children separated from their parents...
...and caged?
I'm not hearing anything. Am I just missing it?
Of all the wonderful stuff the President has worked to address, for me, this horror is the one most important to me.
Does anyone know where we are on this?
I'm seeing a lot more titles in this forum with "Biden" in them than...
...reference too long.orange national nightmare.
I love it.
Isn't it wonderful? The wind is finally blowing in California...
(Graphics in this and previous posts of mine may not be visible in Google Chrome, but should show up in Microsoft Edge, Firefox and Android.)
It appears that all of the wind turbines in the entire state California are producing as much energy as the two nuclear plants operating at Diablo Canyon are producing in two relatively small buildings along the coast.

Source: CAISO Supply Page (Accessed 1/20/21 3:50 pm)
I haven't seen this much wind energy very often in California in recent checks, most of which were around the winter solstice. But I heard that the wind is blowing in California, so hard that trucks are being over turned, so I decided to look.
It's something of a shame that because California is densely crisscrossed by copper power lines to collect all that wonderful so called "renewable energy" that they've had to shut some of those power lines because of, um, wind, and the consequent risk of fire, but one cannot have everything, can one?
As of this writing about 40 minutes later (16:40 PST to be precise) than when I downloaded the above graphic - I was called away to relish President Biden's Press Secretary's first press briefing, a thing of beauty - all of the wind turbines in California are producing 1,563 MW, but don't worry, be happy, at 12:20 they were producing 2,271 MW for a few minutes.
The two remaining nuclear reactors are producing as of this writing, 2,268 MW in two buildings, and have been consistently producing, without interruption, reliably roughly that amount of power all day long, +/- 4 MW.
Solar's been great today, and peaked out at 7,138 MW for a few minutes around 14:30 (2:30 PM) PST, but now, as the sun is falling, is down to 601 MW.
Don't worry, be happy. California, as of 16:45 (4:45) PM is "only" dumping 8,180 metric tons of carbon dioxide an hour to power its (partially shut) grid.
The Press Secretary for the President was very refreshing. She promised something we've been missing, "reality."
This post, by the way, is reality.
We are over 414 ppm of the dangerous fossil fuel waste carbon dioxide in the planetary atmosphere.
Shortly after I started writing here, January 2003, we were at around 375 ppm.
I'm looking forward to a new day, and I hope everyone else is too, but a new day is impossible without a serious day, and we haven't had too many of those.
We are about to have a President, Mr. Joe Biden. So why?
How is it that we have so many posts on DU discussing a lump of shit being loaded on a plane to head for a swamp?
Truth is the daughter of time, not of authority.
-Francis Bacon, as quoted by Dmitri Volkogonov, in Stalin, Triumph and Tragedy
Joe Biden names top geneticist Eric Lander as science adviser
From Nature News: Joe Biden names top geneticist Eric Lander as science adviser Nidhi Subbaraman & Alexandra Witze Nature News January 16, 2021.
It should be open sourced.
Some excerpts:
Many scientists have long called for the OSTP director to be raised to a cabinet-level position. “Having science elevated to its rightful place in the administration seems to me a very positive step,” says Harold Varmus, a cancer researcher at Weill Cornell Medicine in New York City and a former head of the US National Institutes of Health (NIH). “I think it marks a very important moment in the history of science in the government.”
“It signifies the importance of who will be in the room when decisions are being made,” says Roger Pielke Jr, who studies science policy at the University of Colorado Boulder.
Lander was a key figure in the Human Genome Project — the race to sequence the human genome, which ended in 2003 — and is the president and founding director of the Broad Institute of MIT and Harvard in Cambridge, Massachusetts. He will be the first biologist to run the OSTP.
Between 2009 and 2017, he co-chaired the President’s Council of Advisors on Science and Technology (PCAST), an elite panel that advises the US president. Among the PCAST reports issued during Lander’s tenure were some dealing with energy, climate change and vaccine response in the face of pandemic influenza...
… Nobel laureate Frances Arnold, a bioengineer at the California Institute of Technology in Pasadena, and Maria Zuber, a geophysicist at MIT, will co-chair PCAST under Biden. Alondra Nelson, nominated to be deputy director for science and society at the OSTP, is a social scientist at the Institute for Advanced Study in Princeton, New Jersey, who studies genetics, race and other societal issues.
“These are excellent appointments, highly qualified and experienced, and well grounded in science,” Rita Colwell, a microbiologist at the University of Maryland at College Park and a former director of the US National Science Foundation, wrote to Nature in an e-mail...
Tomorrow...tomorrow...tomorrow...it's only 14 hours away...
Some pictures from the process of making coffee.
I was catching up on my reading, and I came across this paper about coffee which claimed that coffee is the second largest consumer product after petroleum: Integrated Design of Biorefineries Based on Spent Coffee Grounds (Manuel Taifouris, Marcos L. Corazza, and Mariano Martín Industrial & Engineering Chemistry Research 2021 60 (1), 494-506).
This seemed like an extraordinary claim, so I decided to look at reference 5 in the paper, on which this claim was based. Reference 5 was this paper: Sustainable management of coffee industry by-products and value addition—A review
I'm not sure the claim is well supported in this paper (although I don't have time to read the entire paper now, but have downloaded for future reference.)
I'm a regular consumer of copious amounts of coffee and in looking at the pictures, I recognized that I have never thought in my life about what goes into the product and whence it comes.
Here's a few pictures of the coffee process beginning with the plant:

The caption:
The plant, which apparently originated in modern day Ethiopia is described like this:
Coffee leaves are opposite decussate on suckers. The leaves appear shiny, wavy, and dark green in color with conspicuous veins. The inflorescence is a condensed cymose type subtended by bracts. Coffee is a short day plant and hence the floral initiation takes place in short day conditions of 8–11 h of day light. Pollination takes place within 6 h after flowering (Fig. 2). Arabica coffee is autogamous with different degrees of natural cross-pollination in contrast to Robusta coffee, which is strictly allogamous with an inbuilt ametophytic system of self-compatibility. The process of fertilization is completed within 24–48 h after pollination. Seeds are elliptical or egg shaped and the seed coat is represented by the silver skin which is also made up of scleroides. The size, thickness or number of pits in the walls of scleroides is considered as important taxonomic characters in differentiating between species. Germination takes place in about 45 days.
Coffee in bloom:

The caption:
Coffee pulping:

The caption:
Coffee drying:

Coffee roasting:

The caption:
A diagram of the coffee "cherry" as obtained from the plant as a fruit:

A schematic of the coffee process with some byproducts:

The caption:
I think it's a good idea to appreciate whence our "stuff" comes.
Pretty cool, I think.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,532