From the Writer of The Simpsons: Nuclear Power and the Simpsons.
Let me start here: Knowing as I do that The Simpsons is involved with the mockery of the nuclear industry - which I emphatically support - I have never watched a full episode of the show.
I was thus surprised to see this article in my Nuclear News Feed: Nuclear power and The Simpsons
(Registration Required; I am, of course, registered.)
The news feed email has this note:
Some excerpts from the full article:
My own interest in fission started back in Columbia, Mo., in the 1970s, when I participated in a fifth-grade classroom debate about nuclear power. Our teacher assigned me to the “pro” side and gave us a few days to prepare. I met with a neighbor down the street—Marc De Chazal, a chemical engineering professor who helped start the nuclear engineering program at the University of Missouri. He was incredibly generous with his time. I recall furiously scribbling notes for more than an hour.
Can you imagine being the kid who had the “con” side of the debate, armed only with a paragraph from the World Book Encyclopedia? He walked into a fusillade of bullet points and data provided by Professor De Chazal, who’d helped build the highest-power university research reactor in the United States. My opponent never knew what hit him. I remember him looking dazed and stammering, “But . . . but . . . there are concerns. Concerns!...”
...So, yes, DOE-NE, we might have taken some liberties.
However, in an episode I wrote in season 33, we mounted something of a defense of the industry. In “Portrait of a Lackey on Fire,” a new business has come to town—a “fast fashion” company—and Mr. Burns says enviously, “Fast fashion is far more toxic than nuclear power.” Mr. Smithers: “It’s . . . worse?” Mr. Burns: “Nuclear energy gives people warmth and light. This guy is profiting off a product nobody needs: a constant stream of brand-new skinnied jeans and be-cropped tops.” We originally had a longer speech about the clean energy benefits of nuclear power but had to cut it for time. One day perhaps that defense will make it onto the show.
I wish I could have sent that Burns scene to Professor De Chazal. I’m sure that his passion and commitment to nuclear energy has inspired many people in more direct ways, but I like to think that he would have gotten a kick out of the idea that his tutelage of a 10-year-old impacted a Simpsons episode 50 years later...
I was surprised, particularly coming from someone who has done so much to ridicule the industry that is the last, best hope of Earth.
Hermit-The-Prog
(33,890 posts)Why isn't it just "fuel we haven't used up yet"?
NNadir
(33,626 posts)In a sense, some components of used nuclear fuel have been put to use.
In the 1960's Soviet scientists powered artic lighthouses with Sr-90 thermoelectrics for instance; plutonium-238 (obtained from neptunium) in used nuclear fuel has powered many space flights and even pacemakers implanted in patients. (The latter have been displaced by lithium batteries.)
The problem, as I see it, has to do with the very small amount of used nuclear fuel that exists, and the failure to reprocess the bulk of it.
For the first year after removal of nuclear fuel rods from a reactor, they do put out significant heat. I often muse that if the used fuel rods at the Fukushima reactor had been placed in a thermoelectric device rather than a cooling pool, the pumps would have not been cut off from power and the operators could have cleaned off the seaweed and restarted the reactors.
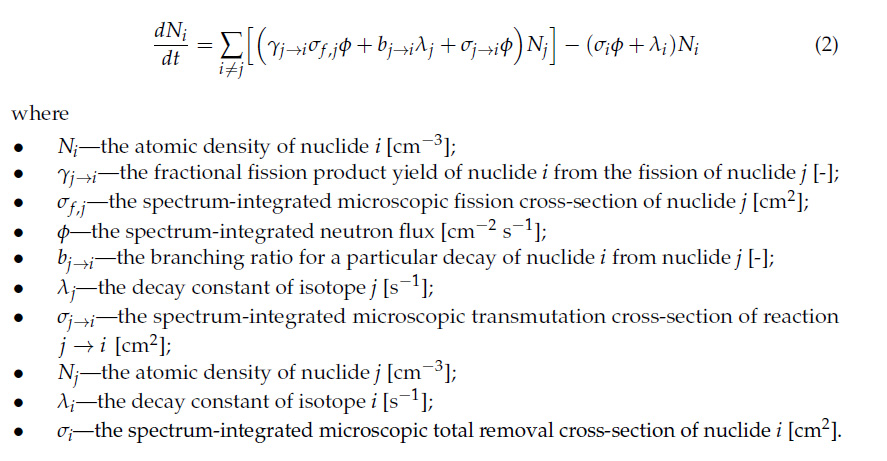
There is another issue with the high heat producing isotopes, strontium, cesium, americium, and especially curium, which is that they are subject to a condition, dictated by a mathematical equation known as the "Bateman Equation," that means they will come to "secular equilibrium," where they are decaying as fast as they form. The exact amount of material that is formed is a function of the overall power levels provided by nuclear energy, as well as the half-lives of the isotopes involved. The isotopes with shorter half-lives provide more specific power than those with long half-lives.
The Bateman equation:
I have been discussing with my son, the budding nuclear engineer, some marvelous (I think they're marvelous at least) ideas for the utilization of radioactive cesium and radioactive strontium in connection with some relatively obscure research reactors that ran in the early 1960s, when the problems they faced did not offer the solutions discovered since. In fact, I chatted with him about this last night when I picked him up from a point on a ride he took with a fellow nuclear engineering Ph.D. student.
In the 1950's there was much active and interesting discussion of utilizing fission products and higher actinides has heat sources and as gamma radiation resources. I access these discussions from time to time, thinking about how the original ideas might be updated.
However to reach industrial scale we need more used nuclear fuel. If our civilization is to survive, if energy becomes sustainable and clean, we will and must have a lot of used nuclear fuel. At that point we will have the resources to put these to use to save what is left to save and restore that which can be restored.
Thanks for your excellent question.