NNadir
NNadir's JournalThe Neuroscience Police.
Interesting, I think.
I also wanted to raise awareness about neuroethics and to learn the opinions of neuroscientists in my own department. But I felt that individual discussions were not enough. I needed something broader — that could reach those who had questions but didn’t know where to begin. I started a podcast: The Neuroethics Police.
I’ve created seven episodes since I started in January 2019, and enjoy the process of creating, interviewing and marketing the podcast, but the road has not been smooth, and I’ve faced several challenges along the way. So if you are planning on creating your own science podcast, I have some insights to share...
Lessons I’ve learnt from creating a science podcast (Nature, Career Column July 4, 2019)
The Neuroethics Police
Can We Recover Carbon Dioxide From the Atmosphere Using Sargassum Seaweed?
I will discuss two scientific papers in this post, one somewhat more extensively than the other. They are:
Biofuel production through micro- and macroalgae pyrolysis – A review of pyrolysis methods and process parameters. (Sara Pourkarimia, Ahmad Hallajisanib, Asghar Alizadehdakhela, and Amideddin Nouralishahib, corrected proof in press, Journal of Analytical and Applied Pyrolysis, 2019)
...and...
Microwave-assisted pyrolysis and analytical fast pyrolysis of macroalgae: product analysis and effect of heating mechanism (Ribhu Gautam, S. Shyam, B. Rajasekhar Reddy, Kasivelu Govindarajub and R. Vinu,* Sustainable Energy and Fuels, Advanced Release, 2019)
All efforts to address climate change have failed, and done so dramatically. It is somewhat ironic that as the crisis is now becoming so obvious that it cannot really stand up to repeated denial, that there has been a world wide renaissance of quasi-fascist or actually fascist ignoramuses advanced into political power in major and not so major countries, including not just the United States, but also the Philippines, Brazil, Hungary, Turkey and most recently Great Britain.
It doesn't bode well.
We, of course, on the left are not innocent in this outcome. We have, in general, although I have personally and certainly been disagreeing vehemently for quite some time, participated in handing out ignorance on our end of the political spectrum, this being the absurd and frankly stupid arguments against nuclear power claiming it's "dangerous" when the death toll associated with nuclear energy is absurdly minuscule when compared with the enormous death toll associated with the dangerous fossil fuel waste in the air, and is about to rise because heat deaths will begin to accelerate and begin to approach the death toll from other forms of dangerous fossil fuel waste and dangerous biomass. These far more deadly forms of energy kill by the formation of particulates, (especially PM10), carcinogens (aromatic PAH's, benzofurans, benzodioxins), carbon monoxide, sulfur oxides, nitrogen oxides (some of which are ozone depletion agents), and let's not forget our friends, the heavy metals, which are an inevitable consequence of fossil fuel combustion. (The world exposure to mercury from vaccines, the target of many ignoramuses, is dwarfed by orders of magnitude by exposure from coal combustion.) These latter types of deaths - excluding heat deaths - are estimated to kill about 7 million people per year, with slightly less than half of them attributed to the combustion of biomass and slightly more than half attributed to dangerous fossil fuels.
The absurdity of the argument that "nuclear power is dangerous" is so stupid by comparison to the obvious health effects of fossil fuels and the obvious effects of climate change from the dangerous fossil fuel waste carbon dioxide, that is should be obvious by inspection but as is the case of other forms of popular ignorance, Trumpism, Brexitism, blah, blah. If I didn't have an ignore button here, I'm sure I'd have to read tripe from these kinds of people, from the kinds of asses who write stuff - this actually happened - about the collapse of a tunnel at the Hanford weapons plant being a tragedy, while the heat deaths across Europe is regrettable but acceptable.
These kinds of people actually exist. Incredible, but they do.
Anyway, despite my hostility to so called "renewable energy" - such hostility arising from the fact that these fantasies, despite absorbing money on a multi-trillion dollar scale they have not done a damned thing to address climate change, are not doing any to address climate change and won't do anything to address climate change, I am about to write about biofuels (and while not explicitly stated) biomaterials.
Right now, not in some far off future getting the attention of the likes of say, Bill McKibben, anti-nuke "concerned" about climate change, or other weak minded people like say, Joe Romm, another shit for brains anti-nuke "concerned" about climate change, a massive mat of the macroalgae known as Sargassum, which used to be fairly limited to the Sargasso Sea in the Atlantic, now stretches across the entire ocean. While such outbreaks have a huge potential to kill the seas by the same mechanism that has killed the ecology of the Mississippi River Delta, by depleting oxygen when the algae dies and is decomposed by bacteria, it occurs to me that these macroalgae contain a lot of carbon, from the world's largest, by far, dump for the dangerous fossil fuel waste carbon dioxide, the ocean.
So I decided to look for recent articles - limiting the search to 2019 - to studies of the pyrolysis of sargassum, picking, at random, the two aforementioned papers from the collection I was able to download in about 10 minutes time.
"Pyrolysis" is the practice of heating mixtures of carbon compounds - biomass is an obvious case of such a mixture, although there are others, waste plastics for instance - in the absence of air, or at least oxygen, a temperatures at which they decompose to useful chemicals. This is hardly a new process. A very long time ago, when I was a child, one could buy in hardware stores cans of "wood alcohol" which was essentially methanol, the C1 alcohol, H3COH. Methanol used to be made by the pyrolysis of wood, although today it is largely made from carbon monoxide obtained from dangerous natural gas.
Although one can obtain biofuels from a number of processes, the silliest being one of the most widely used, fermentation, a water and energy intensive process, other processes exist and in my view pyrolysis and the related practice of supercritical water or supercritical carbon dioxide oxidation are, unlike every other so called "renewable energy" scheme, possibly sustainable (under the right conditions) and in fact are the low hanging fruit for the removal of carbon dioxide from the atmosphere. This removal of carbon dioxide, an incredible engineering challenge, may prove a matter of survival for future generations, about whom we clearly couldn't care less while we blithely carry on mindlessly about how they'll all drive Tesla cars some day.
The processes for obtaining biofuels (and more importantly biomaterials although they are scarcely mentioned in these papers) are shown in this graphic from the first paper cited:

The caption:
Here by the way, is an excerpt from the authors' introductory text in this review paper:
Third generation biofuels mainly derived from algal biomass are the most promising alternative resource without the drawbacks of first- and second-generation biofuels [14]. Algae are simple aquatic organisms living in fresh or saline water and are able to convert sunlight, water, and carbon dioxide into algal biomass photosynthetically. Due to many advantages, such a fast growth rate, CO2 fixation ability, possibility to grow in non-arable lands or in saline and wastewaters and no competition with food/feed crops, algal biomasses appears to be ideal third generation biofuel feedstocks [15]. However, large-scale production of algal biomass and cost efficiency generation of final bio-fuel products are the main problems associated with these biomass resources. It has been estimated that the average cost of biodiesel generated from microalgae is seven times higher than the current price of diesel [13]. Conversion of algal biomass into valuable energy products can be performed mainly by thermochemical, biochemical and chemical pathways [16,17]. Thermochemical conversion techniques use heat and chemical catalysts to produce the energy in three forms of bio-oil, biochar, and gaseous products while chemical/biochemical processes form mainly bio-diesel, ethanol and hydrogen [4,18]. Among thermochemical conversion processes, pyrolysis is a popular approach wherein the thermal degradation of the biomass components is performed in an inert atmosphere [19]. Pyrolyzed products in the form of bio-oil, biochar and gas can be used as fuel for energy generation. Besides fuel resource, biochar can be effectively used in agriculture as fertilizer to upgrade yield and plant nutrition [20–22], and in other fields to remove heavy metals [23–27], dyes [28] and other organic, inorganic and microbial pollutants [29,30].
I will focus in this discussion of sargassum for two reasons, one being that it is here in now disturbing quantities and the other being that it, like coral, tends to concentrate uranium from seawater, (along with other metals), the quantities of uranium being present in seawater is on the order of five billion tons. This means that the sargassum - given its huge mass - is a potential low grade (but workable) uranium ore when the carbon is removed from it. Thus potentially it contains the enormously high energy density fuel that might be used to pyrolyze it, much of the cost of pyrolysis being driven by the heat to affect it. Also sargassum is a macroalgae, meaning that it is easy to remove from seawater with simple nets; it also is likely to capture mechanically or internally, large amounts of the plastic waste that now contaminates the ocean.
If you don't know what sargassum is, here's a picture of it:

If you've been to the ocean, you've probably seen it lots of times.
The elemental composition by weight of sargassum is given in this first paper, 25.9% carbon, 5.57% hydrogen, 24.18% oxygen, 3.58% nitrogen, 1.22% sulfur. Among species of algae, it has the lowest high heating value, 8.68 MJ/kg, but this should not really matter unless the goal were direct combustion, a dangerous process I would oppose.
In terms of the biochemistry, sargassum is said to be (in this paper) about 10.25% protein, 0.74 lipids, and 41.81% carbohydrates. Possibly the balance, not specified, is represented by lignins. The low lipid content accounts for the low heating value, which means this particular species would not be good for producing biodiesel, although there is no particular advantage of biodiesel compared to other potential fuels, particularly those derived from carbohydrates, notably the furans, and, at higher temperatures, particular in supercritical water, carbon monoxide and hydrogen, syn gas, a mixture from which pretty much any commodity now provided by petroleum can be synthesized, and commodities superior to gasoline like dimethyl ether can also be synthesized.
This graphic from the first paper gives a scheme for chemicals and fuels that can be obtained from pyrolysis:

The caption:
I personally find this graphic unsatisfying - but I picked this paper for this discussion somewhat at random - because it does not discuss biomaterials, specifically the many important allotropes of carbon itself, nor does it discuss carbides. These allotropes and carbides represent the opportunity to sequester carbon at a profit.
A process schematic, one very limited in scope:

The caption:
Fixed bed reactors more or less imply batch processing, which is inherently more expensive than continuous flow systems.
Fluidized bed reactors can approach continuous processing a little closer; here's a schematic of one:

The caption:
According to the authors, the following situation applies:
I'm not sure I necessarily buy this statement - I favor syn gas above all other options - since I'm not sure it includes some consideration of very high temperatures, nor does it consider conducting reactions in supercritical oxidation systems using supercritical carbon dioxide or supercritical water.
The second paper focuses more closely on sargassum, and it is therefore more relevant to the situation now covering much of the atlantic ocean.
From the introduction:
The scientists here are Indian nationals; the previous paper was written by Iranian nationals.
The graphics in this paper address more directly what sargassum can do and the conditions for doing it:
I'll just fire these graphics off as a set:

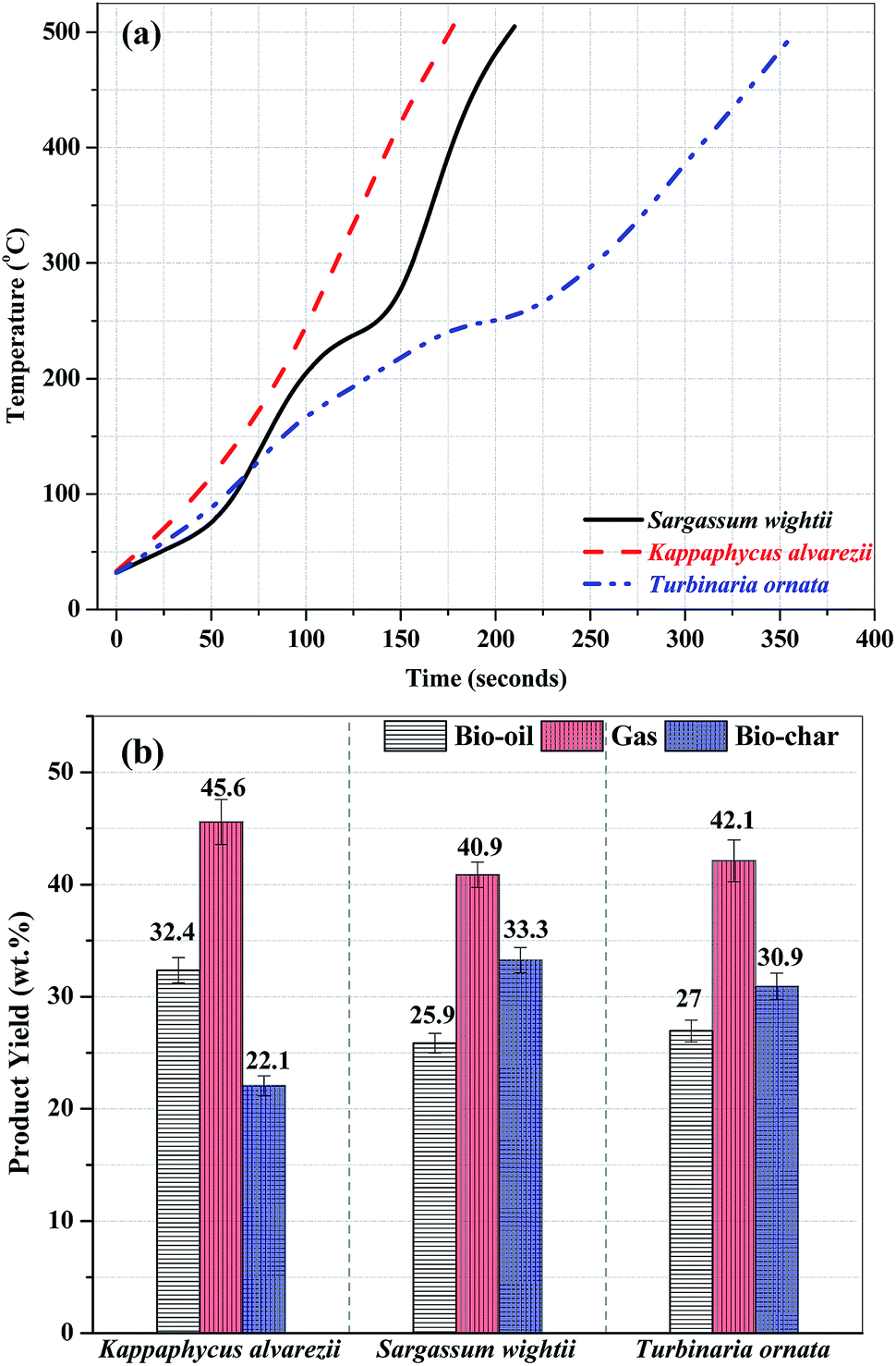
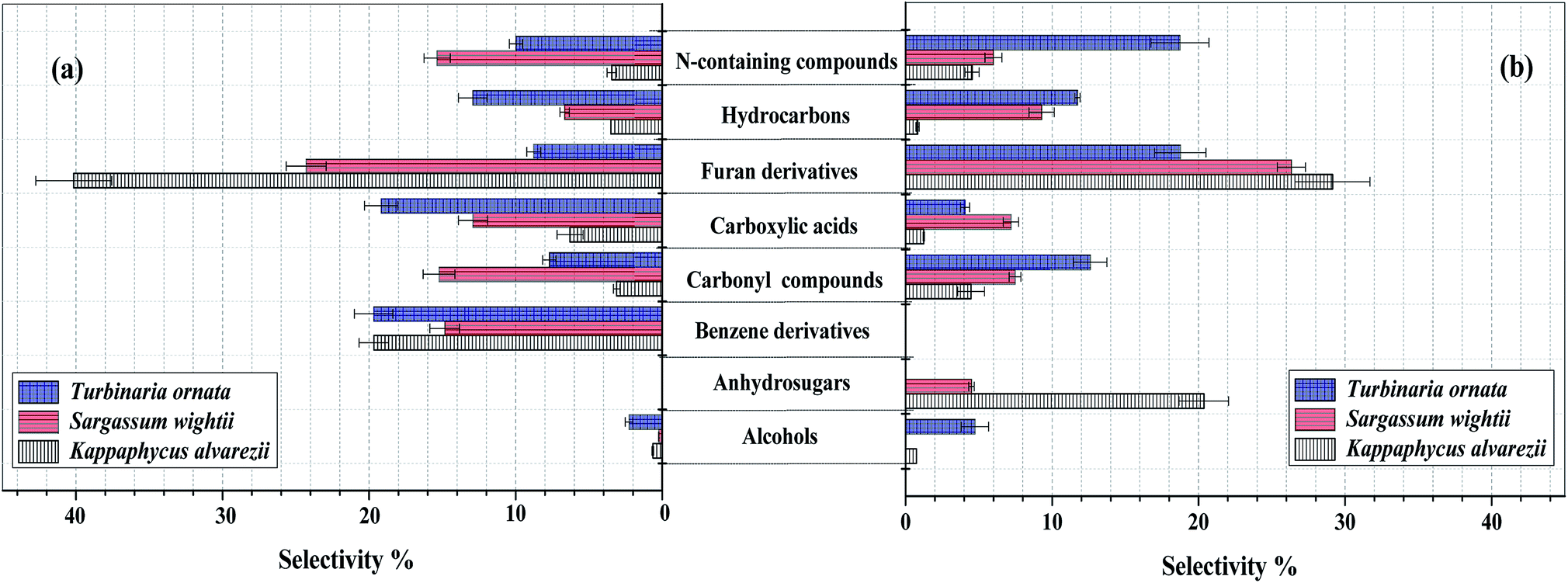
Fig. 3 (a). Composition of bio-oil from microwave-assisted pyrolysis of different macroalgae at 500 °C. (b) Composition of the pyrolysates from Py-GC/MS of different macroalgae at 500 °C. The data are presented as the mean ± S.D., n = 3.

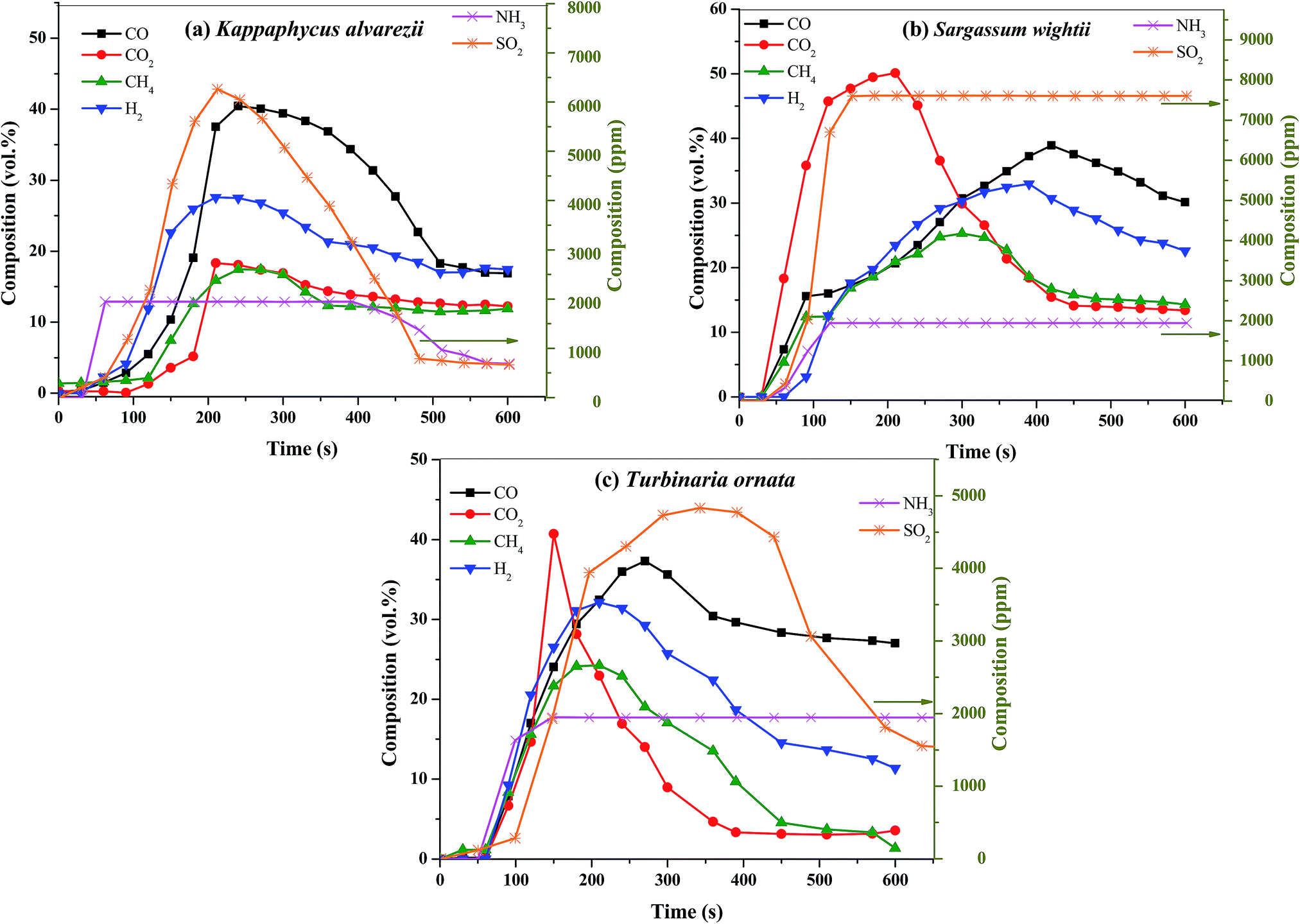
Note that the heat source here is microwaves, and the temperature is rather low for my tastes, 500 °C. (There is brief mention of higher temperatures.)
Microwaves are featured because of their fast heating rate, but continuous heating can be achieved by gas flows, particularly with nuclear heat, which is of course not mentioned here.
Many of the chemicals shown would require further processing to make them usable as fuels, for example the furans, or as commodity chemicals. The alkanes are linear, and thus not really "gasoline-like" which is not a bad thing necessarily, since gasoline is a ridiculously dangerous and noxious fuel, one that we stupidly accept because of unwarranted (and dangerous) familiarity.
Full oxidation to syn gas is discussed only briefly, and then in the context of co-existing with bio-oils, many of which are phenolic species, somewhat corrosive and somewhat unstable oils. Here is an excerpt focusing on syn gas:
Sargassum contains less sulfur than the other two species investigated here. It also produced higher amounts of furans, which are potentially direct use biofuels, but more importantly, can be utilized for the production of certain kinds of interesting polymers which, in long term uses (as opposed to the noxious practice in which most of us are more or less forced to participate, single use plastics) represent carbon sequestered from the air, indirectly via seawater.
(Syngas, via the Boudouard reaction after hydrogen removal, can be made into carbides.)
I know I'm rambling here, and I'm tired, and will cut it here, but this type of process is interesting, given the sargassum catastrophe in the Atlantic ocean which will get worse before it gets better. It is only really useful if the sargassum is mined using nuclear powered ships - we know well how to make these - and pyrolyzed using nuclear reactors.
Without this, it's probably just another useless treadmill, like the rest of the so called "renewable energy" schemes.
Enjoy the rest of the workweek.
Slow dancing in a burning room.
?list=RDEMbkyPiFogY0Mw3NR759WCbQWar Requiem.
Seeking Life's Origins, a Non-Enzymatic Route to Peptide Synthesis In Water Is Discovered.
The paper I'll discuss in this post is this one: Peptide ligation by chemoselective aminonitrile coupling in water (Powner, Islam, and Canavelli, Nature 571, 546–549 (2019))
Great fundamental scientific questions remain mysterious, and among the greatest is the question of how life came to exist, and by extension, how we came to exist. The great strides in molecular biology that began accelerating rapidly in the 1980's up to the present day have come so far that we are now at the cusp of being able to design life, even synthesize life, but the question still remains about how life arose spontaneously, without a designer, given the great complexity that life now exhibits as a set of closely interacting highly complex molecules.
(Please note that this statement does not imply that the absence of an extant explanation does not imply intelligent design anymore than a lack of an explanation for the heat of the sun, which was a complete mystery until the relatively recent discovery of nuclear reactions, implied the necessity for the existence of Apollo. A question lacking an answer does not imply an arbitrary answer must be true.)
Many interesting theories have been advanced, going back to the Miller experiment in the 1950's, where amino acids, the constituents of proteins, were synthesized from a putative mixture that was believed to represent the Earth's early atmosphere subjected to electrical discharges (lab lightening) and high energy radiation. However these amino acids lacked the asymmetry that characterized all living things we observe today (with some rare exceptions to the case) and the origin of asymmetry itself remains as much a question as the origin of the sun's heat presented as late as the 1930's.
It is now known that asymmetric molecules exist in meteorites suggesting they are available outside of Earth bound processes; it may be that this asymmetry may be tied to certain asymmetries observed in nuclear reactions. It is also known that purines and pyrimidines, which represent the cores of nucleic acids are also found in space, and there is some evidence that primitive sugars - sugars also essential to nucleic acids - also exist in space. The Nobel Prize generating discovery that RNA could do what proteins do, catalyze chemical reactions (awarded to Thomas Cech and Sydney Altma) suggested evidence - but not proof - "chicken and egg" argument, since the formation of proteins is dictated by nucleic acids in living systems now observed, and the synthesis of nucleic acids like RNA and DNA catalyzed by proteins.
One of the greatest mysteries is how amino acids can couple, form peptide bonds in water. Human beings can now couple amino acids on an industrial scale - I've participated in such projects - and peptides, sequences of amino acids that make up proteins having scores of such bonds have been synthesized on a ton scale. The chemistry however under which this is done avoids water, in fact can be said to remove water, and is often conducted using solvents and reagents that would be quite hostile to living systems, and in any case, is unlikely in a prebiotic world.
This brings us to the paper evoked at the outset of this post; by looking at some artifacts of how life still operates, using sulfur based oxidation, the authors have synthesized peptide bonds in water.
From the abstract:
From the introductory body of the paper:
In organic synthesis, a route sometimes used to synthesize carboxylic acids (which amino acids are) proceeds through cyanide, and in fact, an early synthetic route to amino acids, known as the Strecker synthesis is a reaction involving cyanide:

Asymmetric Strecker syntheses of amino acids is well known - I've run many of these myself - but these rely on asymmetric catalysts the ultimate source of which is living things.
The paper continues:
[div class="excerpt"...]Maurel and Orgel have previously suggested that AA-SH16 might offer an interesting alternative to biological thioesters10,11. AA-SH combine excellent aqueous stability with highly selective (electrophilic or oxidative) activation12,14,16,24, but their prebiotic synthesis presents difficulties25 and they undergo inefficient ligation at near-neutral pH (Supplementary Discussion)16,26. To overcome these problems we reconsidered the synthesis of thioacids from nitriles (Fig. 1b). Recently we reported that high-yielding nucleophilic displacement of sulfides by Gly-CN19 is promoted by the low pKaH of AA-CN in water, and we hypothesized that coupling AA-CN to the C terminus of a growing peptide would be facile at neutral pH...
The pictures:
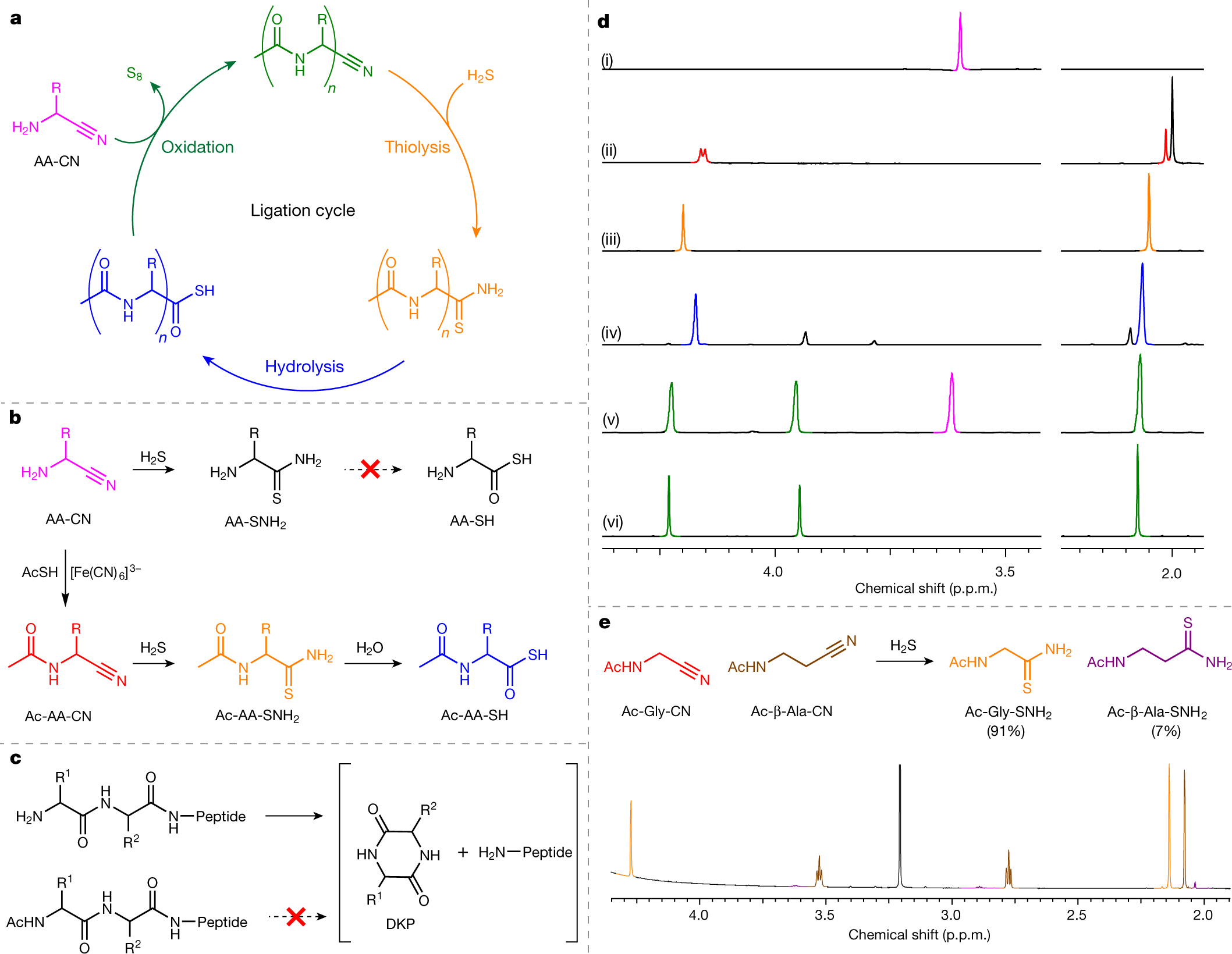
The caption:
There is a fair amount of text in the paper detailing the process, including a well known complex of cyanide, the iron complex ferricyanide (a constituent of the pigment Prussian Blue), but overall the paper suggests a route to prebiotic uncatalyzed peptide synthesis, and as peptides make up proteins - the distinction between a large peptide and a small protein is not entirely well defined - this adds some interesting clues.
From the paper's conclusion:
Interesting work, I think, perhaps somewhat esoteric, but in these trivializing times, uplifting to see.
Have a nice day tomorrow.
Muscle Shoals Rhythm Section.
Nice interview with Mayor Pete in a book store.
I came across it in the Buttigieg forum, where I cannot post since he is not my preferred first chance candidate:
Pete's Feb 2019 interview at Politics and Prose bookstore (DC)
The direct link is here:
The Shortest Way Home
He comes across very definitely a fine and thoughtful young man, a bit too technocratic perhaps - and I say this as a technical guy - but very much aware of the limits of technocracy.
Were he the nominee, I would enthusiastically vote for him, and if he's not the nominee, which I suspect he won't be, I do hope he will be a high government official in a Democratic Administration.
We really have a wonderful field overall.
My son made me go bicycling this morning with him, with the heat already rising.
It was already well into the 80's Fahrenheit.
I love that young man.
He's so damned good for me.
I feel much better, particularly in my diseased old man hip.
I support Elizabeth Warren because, according to the NYT puff piece, she used to be a Republican.
It was in the New York Times Magazine a few weeks ago. It's unsurprising in a sense, that she was once a Republican; she was born into the white lower class in Oklahoma.
I am, of course, proud that I have never voted Republican in my life, although once as a kid, I toyed with voting for Jacob Javits in the Senate but didn't, because he was a Republican.
But the point is not about whether I have ever thought that Republican "ideas" - if we can call them that - were worthy of consideration.
The point is that as a young woman, Ms. Warren assumed they were.
But there's this: Elizabeth Warren as an academic researcher began to do research on the premise that people filing for bankruptcies were involved in a kind of credit card scam, living beyond their means and then leaving the banks holding the bag for their high living lifestyle. And in her research she found something she didn't expect; that the majority of bankruptcies involved unexpected medical expenses, lost jobs, other kinds of tragedy.
So she changed her mind. She let go of her hypothesis and engaged the reality she found.
And that's precisely what I want in a President; high intelligence coupled with a flexible mind.
She's traveled a greater distance that I have and I admire this.
I will vote for the Democratic nominee; but I hope it will be Warren, and if not Warren, I hope the person to humiliate that racist sexist piece of crap in the White House will be a woman; and if not a woman, well, I'll take any of the fine candidates we have before us.
But I have surprised myself with this reason for supporting Ms. Warren in the present state. It's something I thought I'd never say.
Correcting datasets leads to more homogeneous early-twentieth-century sea surface warming.
The paper I'll discuss in this post is this one: Correcting datasets leads to more homogeneous early-twentieth-century sea surface warming (Huyber et al Nature 571, 393–397 (2019))
It was really not all that long ago in history that the concept of "temperature" was qualitative as opposed to quantitative; one knew it was, for example, "hot" but how hot in comparison to previous "hot" was not clear. Calibration to international standards has improved greatly in the last 50 years, but the standardization of conditions under which measurements were made varied considerably.
We have chosen to destroy the planetary atmosphere for short term gain, and for those of us who think this may have been a bad, as in immoral, idea, it is important to make temporal comparisons. The problem with doing so is that one cannot really discern how the measurements were made in order to make accurate comparisons.
The paper under discussion is an attempt to adjust for that, to find out if there really are regions of the oceans that are cooling rather than heating. Although it is well known and well understood that the planet as a whole is in a death spiral of heat - Elon Musk's wonderful car for rich people and all the delusional marketing pictures of wind turbines and solar cells notwithstanding - and the that the average overall temperatures are climbing, the situation with respect to certain local areas has not been as clear.
The paper is an effort to address that point by taking a close look at the important issue of accuracy.
From the abstract, which is open sourced:
From the body of the text:
nother possibility is that observational estimates of SST changes contain undetected biases, for which there are some precedents. Difficulty in simulating a slowdown in global warming between 1997 and 2012 was partly reconciled by revising SST estimates19, amongst other considerations20. In another study21, a jump in global temperature by 0.3?°C in 1945 was attributed to offsets between engine-room intake and bucket SST estimates.
The four major SST products covering the early twentieth century each rely upon the International Comprehensive Ocean-Atmosphere Data Set (ICOADS)22, whose latest release is 3.0. It is estimated that 94% of observations between 1908 and 1941 were from buckets (Fig. 1). Bucket measurements of SST are biased by evaporative, sensible and solar heat fluxes that depend on a range of factors, including weather, ship deck height and bucket type7. For example, a canvas bucket left on deck for three minutes under typical wind and other weather conditions can give water temperatures that are approximately 0.5?°C cooler than a wooden bucket measured using the same protocol7,9...
Some graphics from the paper:
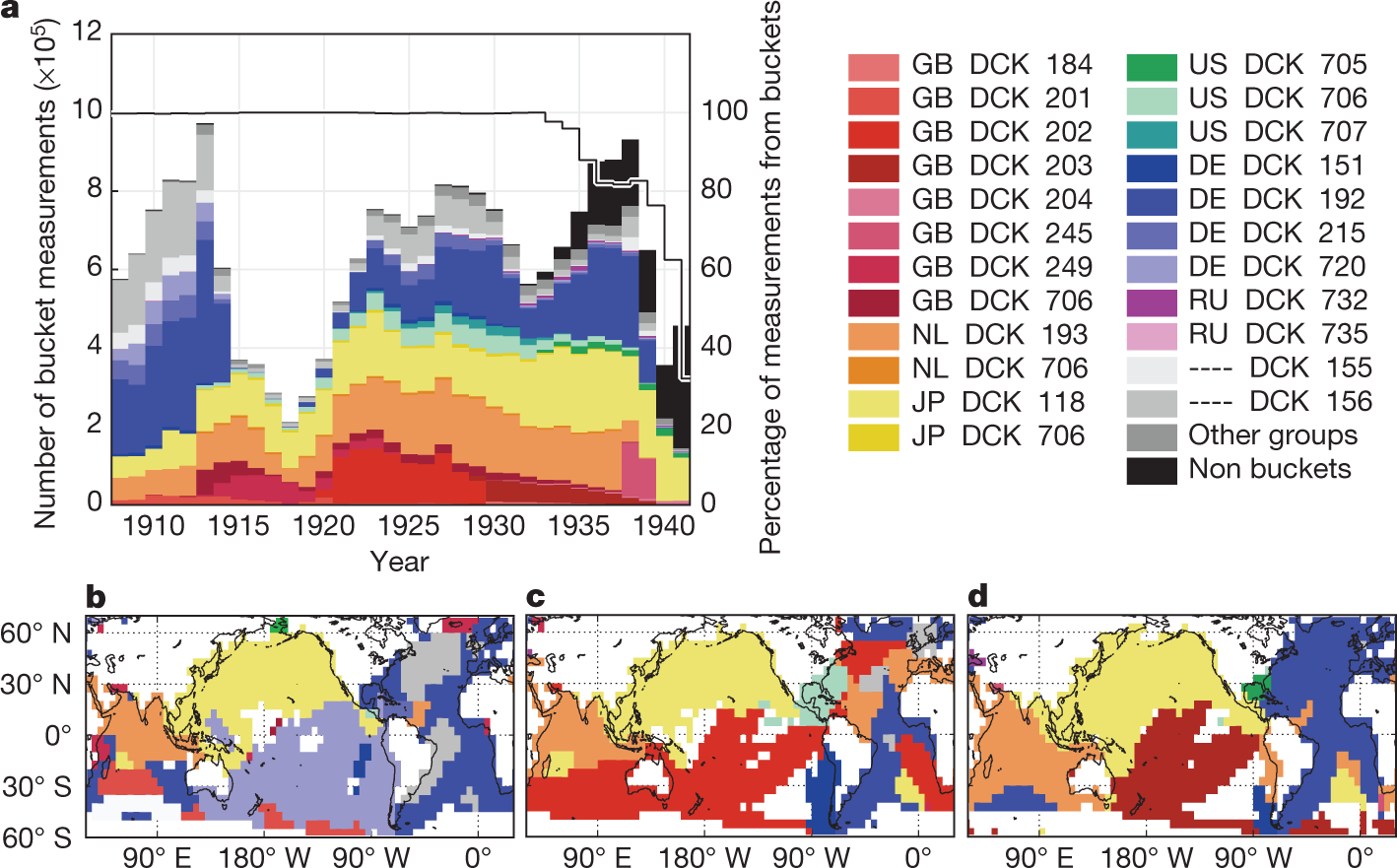 ?
?
The caption:
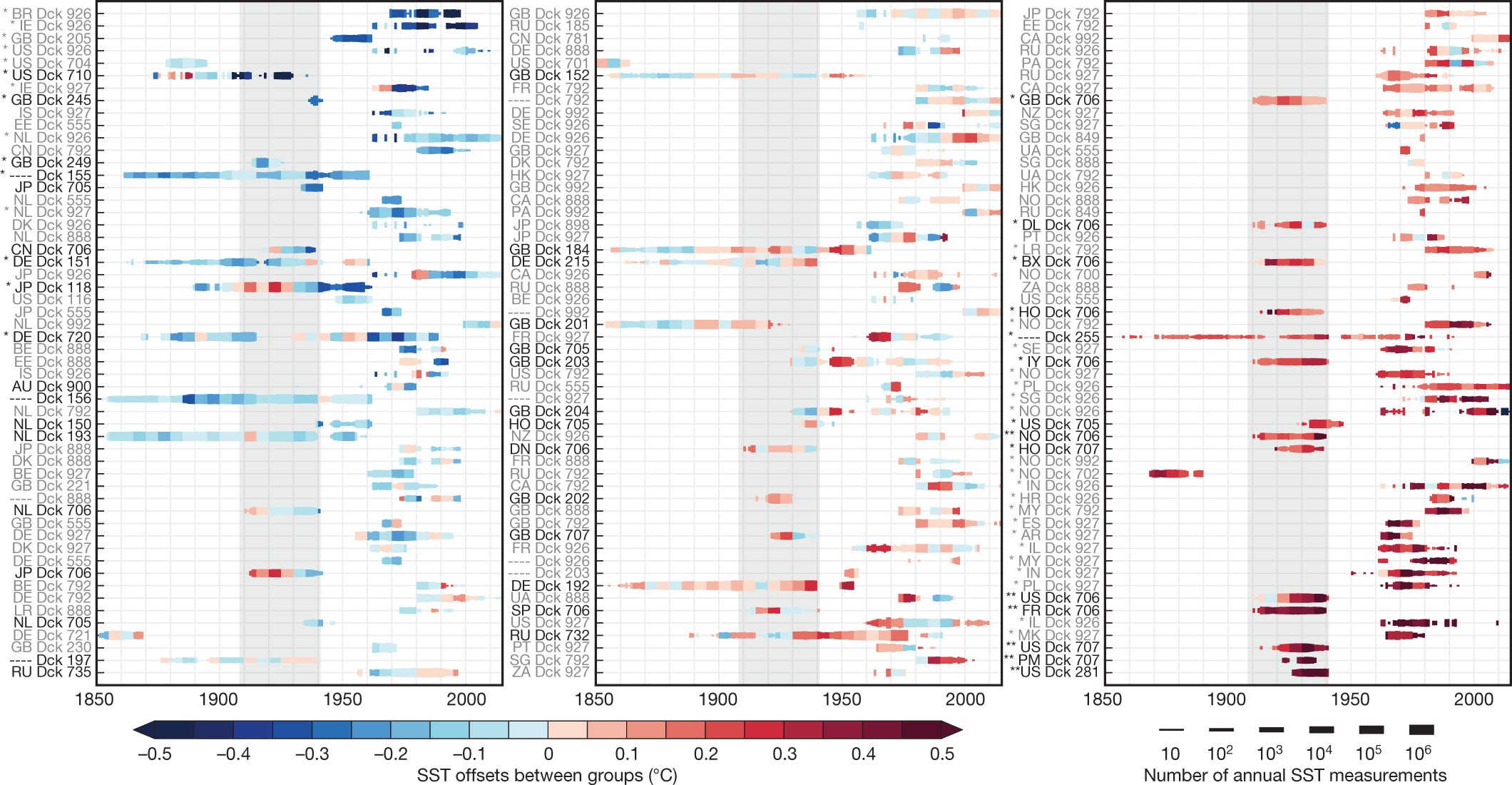
The caption:
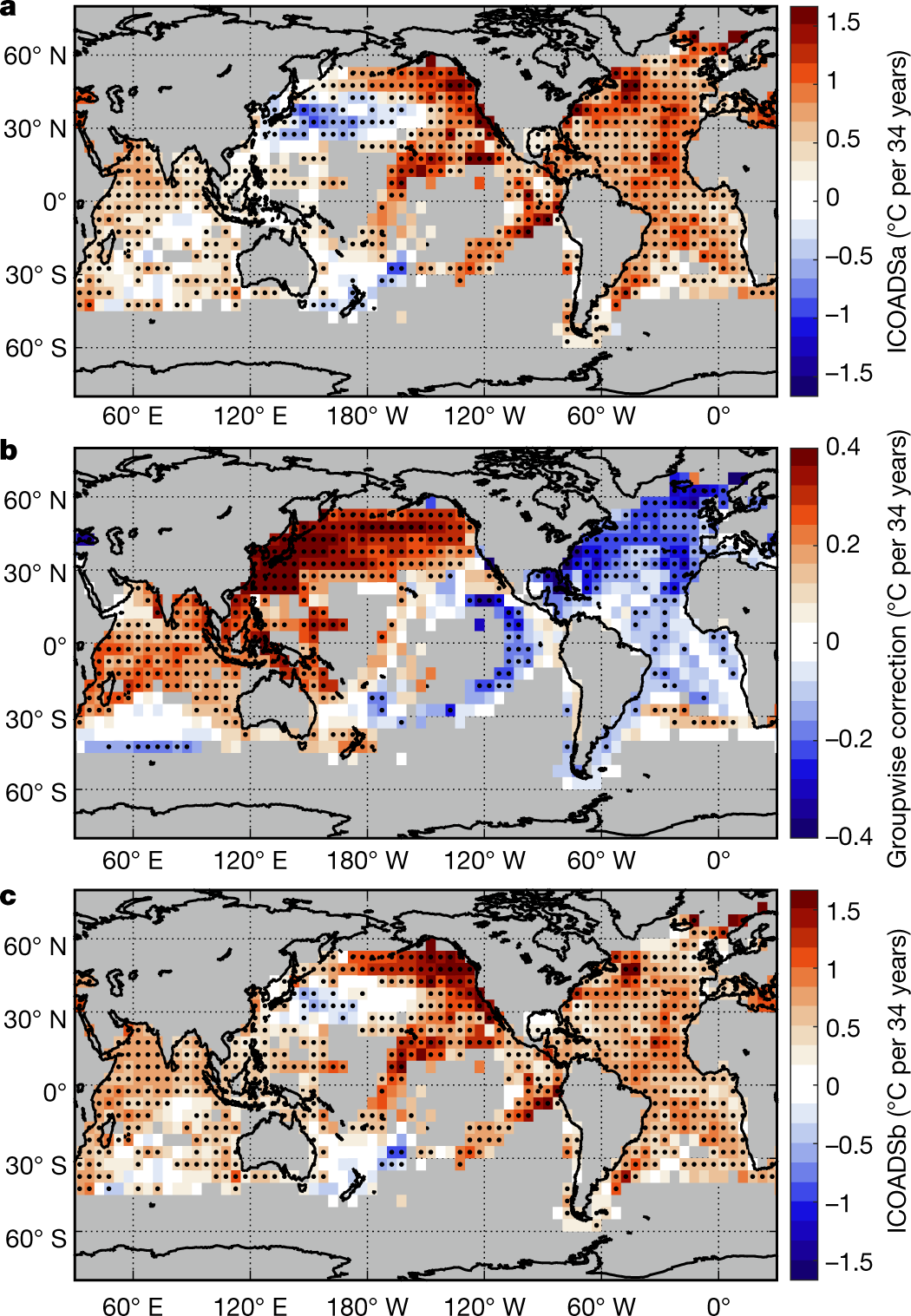
The caption:
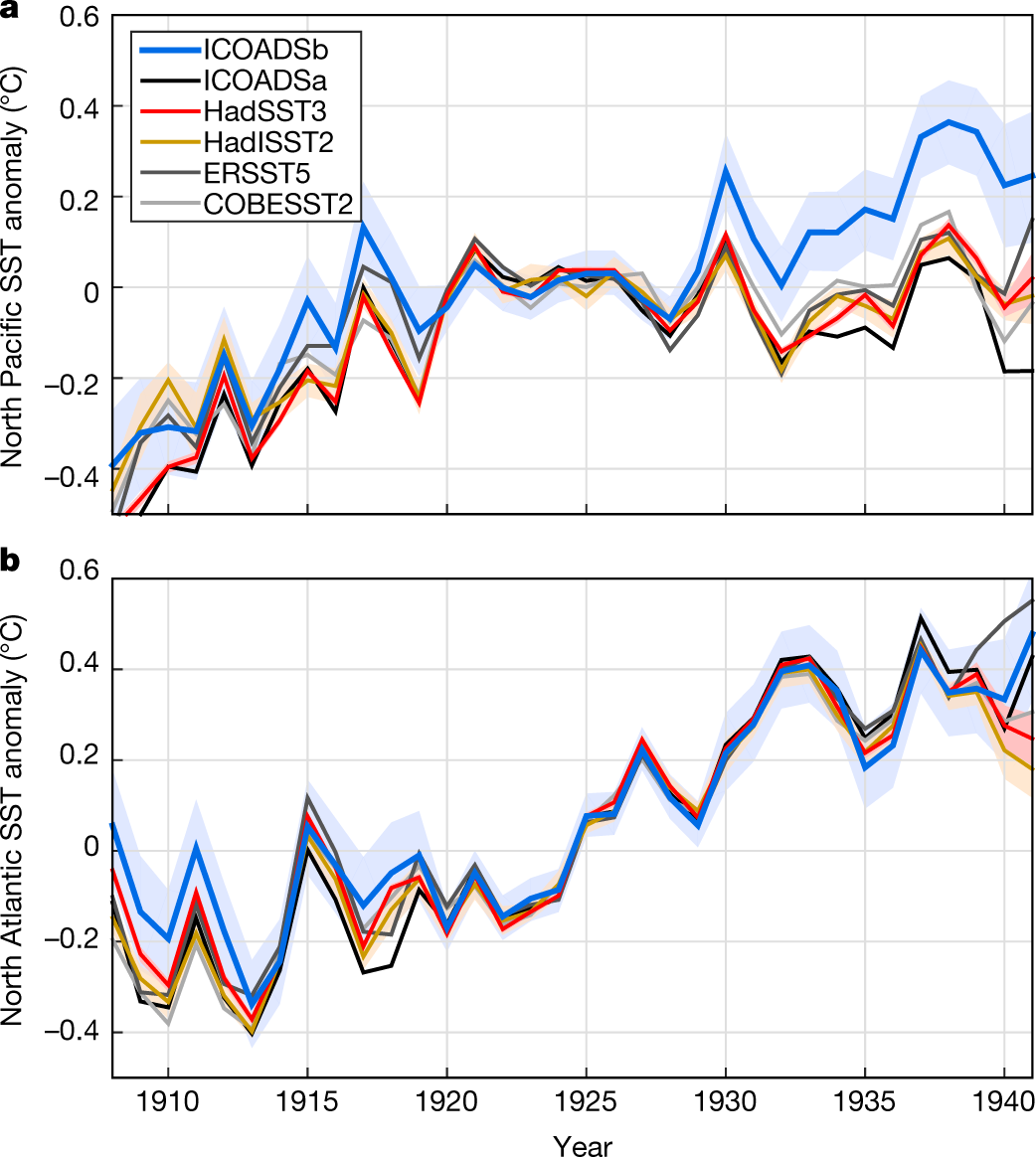
The caption:
From the conclusion:
Maybe things are worse than we think, although at 415 ppm CO2 levels having been hit this year, things are clearly pretty bad.
The 2003 European heatwave is said to have killed 70,000 people, upon analysis.
Death toll exceeded 70,000 in Europe during the summer of 2003 (Plus de 70 000 décès en Europe au cours de l'été 2003) (Robine et al Comptes Rendus Biologies
Volume 331, Issue 2, February 2008, Pages 171-178.)
Global warming is an issue involved dangerous fossil fuel waste.
Still, at this point, including 2019, the death toll from heat has yet to rival the death toll from exposure to dangerous fossil fuel combustion wastes and dangerous biomass combustion wastes, aka air pollution, more than 7 million deaths per year.
It's too bad of course, that our knee jerk discussions of energy wastes tends to focus on so called "dangerous nuclear waste" even though the "dangerous" adjective is applied by air heads who cannot point to a death toll on the order of 70,000 deaths over the whole life time of commercial nuclear energy, never mind a single summer, and nothing like the 70,000,000 people who died in the last ten years from dangerous fossil fuel and biomass combustion wastes.
Tell me again, so I can understand it, just how dangerous so called "nuclear waste" is in comparison. )Only numerical historical data can be worthy of respect, wild-assed paranoid speculations don't count.)
We live on an insane planet, and not all of the insanity derives from the ugly orange ignoramus racist in the White House.
Have a nice weekend.
Profile Information
Gender: MaleCurrent location: New Jersey
Member since: 2002
Number of posts: 33,509