Science
Related: About this forumExtraction of Desalinated Water from Brine with Diisopropyl and Tripropyl amines.
The paper I'll discuss in this post is this one: Molecular Simulation of High-Salinity Brines in Contact with Diisopropylamine and Tripropylamine Solvents (Praveenkumar Sappidi, Gabriel Barbosa, Brooks D. Rabideau, Steven T. Weinman, and C. Heath Turner Industrial & Engineering Chemistry Research 2021 60 (21), 7917-7925)
It is abundantly clear that all efforts to address climate change have failed, despite lots of rhetoric and websites, and big Live Earth rock concerts with hirsute heavy metal guitarists wearing "No Nukes" tee shirts, and Bill McKibben's 350.org website and Greenpeace websites featuring protests with people wearing monkey suits or rock climbers hammering pitons into the sheer faces of huge landmarks to hang insipid banners as part of puerile bourgeois "protests," and slick corporate ads about electric cars showing wind turbines and solar farms in the background.
I personally find this unsurprising, but that's just me. Everybody else seems to be still gushing interminably over "going green," although if one really looks, it takes very minimal effort to see that things are getting worse, much worse, not better.
This said, we live in the age in which lies are celebrated as lies, and the lies we love best are those we tell ourselves.
The waste from these expensive efforts of no result, the effort "to go green," will remain with humanity, basically, forever. This includes the stuff that will leach out of all the fracking holes we drilled to get "transitional" dangerous natural gas that was never, not ever, really "transitional" at all. Beyond that, the purpose of my generation was to consume all of the world's best ores, as much of the dangerous fossil fuels as possible, all of the land, all of the forests, all the best copper ores, all of the indium, all of the dysprosium, all of the neodymium, all the praseodymium, all of the cobalt so we could declare ourselves "green," and "sustainable."
Above all of the things we have robbed from all future generations, in full and barely obscured contempt for them, this on a planet dominated by seas and oceans, is clean fresh water. Many of the shortages of fresh water are connected with climate change, others ironically are the function of impeded riverine flows, still others relate to the poisoning of water sources, in particular, ground water with industrial, agricultural and consumer chemicals, and especially in the age of fracking, but not limited to it, mining.
Some years ago I was trying to write an elaborate intricate arcane novel about unhappy doomed people, and to do this in a kind of isolation, I'd spend an hour every morning in a little coffee shop in my town. Ultimately the coffee shop shut, driven out of business by rent and Starbucks, and the worthless novel was put away for other reasons, but toward the end of the affair I sometimes found myself chatting with the owner of the coffee shop, who sold great coffee, nice high end chess sets, and very snazzy neck-ties. Somehow the owner gleaned that I knew a lot of science, and one day, during a terrible drought in New Jersey that was killing old magnificent trees and lots of other things, the owner asked "Why don't we just desalinate ocean water."
Why don't we just...?
I probably gave him a one word answer, which was "Energy," but the truth is that I read a great deal about the state of water on this planet, and I think all the time about ways to save it or restore it, and I believe I've written in this space on this subject a number of times, although I do of late, as life winds down, lose track of my own drivel as it becomes more spitting into the wind.
My favorite scheme for water purification using seawater is phase separation under supercritical conditions - for which there is a set of problems for which I can at least imagine solutions - but there is always of course, distillation, or flash distillation, albeit involving materials science issues similar to the supercritical case, although large water distillation desalination plants have been built. Of course, membrane methods also exist and are probably the most commonly practiced method, often described as "reverse osmosis" although there are "forward osmosis" schemes as well. These have different sets of problems.
I read a lot, but somehow I never came across a method like the one being modeled in the paper referenced at the outset, solvent extraction of water from brine.
So I thought I'd write a little bit about it here, since sometimes threads at DU serve me as kinds of "Post-it" notes.
The introduction to the cited paper focuses heavily on water contaminated by mining processes, particularly on "transitional" natural gas (and oil). (The growth in use of "transitional" natural gas is easily outstripping so called "renewable energy" (measured in units of energy as opposed to putative "peak" capacity) and has been doing so for decades.)
An excerpt from the beginning of the introduction:
Reference 8 is a short 2004 National Academy Press Book, which is available for free download: Review of the Desalination and Water Purification Technology Roadmap
It contains this deliciously hopeful text:
We're past "by 2020;" it's widely reported that this is 2021, one year after "by 2020." Are we there yet? How about all the "entirely new" energy sources. In 2004, this statement was presumably about so called "renewable energy," and was before trillion dollar "investments" were made in solar and wind energy with no meaningful effect on even slowing the degradation of the entire planetary environment.
If one is in the habit of making predictions, it is useful to look a predictions made many years ago. We generally don't do that. When I do it, I generally meet with an uncomfortable mixture of amusement, cynicism, and outright disgust. It's why, even though I'm a political liberal who actually cares about the future of the planet, I am completely disabused of my former, de rigueur (among political liberals) enthusiasm for solar and wind energy. I hope I'm not being too egotistical in claiming I am familiar with something called "critical thinking."
The excerpted introduction to the paper continues:
...Overall, solvent extractive desalination (SED) has several advantages, such as the lack of a membrane (minimizing maintenance), moderate operating temperatures (40–60 °C), and solvent recyclability after water decantation.
Rish et al.(16) performed SED experiments using DA as the solvent and showed salt rejection rates as high as 98–99%, which is equivalent to RO performance. Guo et al.(17) also performed SED experiments and simulations using DA as the solvent for separating arsenic from contaminated wastewater, and the demonstrated separation efficiencies were as high as 91% for As(III) and 97% for As(V)...
...Also, Boo et al.(19) used diisopropylamine (DiPA) as a solvent for temperature swing solvent extraction (TSSE) for zero liquid discharge, and they found that DiPA is a potential candidate for extracting water from high-salinity brines, with total dissolved solids as high as ?292 g/L.
Reference 19 is this one: Zero Liquid Discharge of Ultrahigh-Salinity Brines with Temperature Swing Solvent Extraction (Chanhee Boo, Ian H. Billinge, Xi Chen, Kinnari M. Shah, and Ngai Yin Yip Environmental Science & Technology 2020 54 (14), 9124-9131) I looked at my notes from when I reviewed this issue of this journal - I review the Table of Contents of every issue of it - and I seem not to have noted this paper. I can be such a slob at times.
Despite the "foreign sounding" names of the scientist authors of this paper, reference 19 - all of whom are vastly more intelligent than redneck anti-immigrant morons like Ted Cruz, Josh Hawley, Ron Johnson and their fans in the White Supremacy Party - they are at Columbia University in New York, although they discuss environmental policy in China. Smug Americans, who like to brag about "being green," like to make fun of environmental policies in China, although their law on brine discharge may be compared with American laws on Brine discharge from fracking operations which basically don't exist:
I have added the bold.
About the moderate operating temperatures (40–60 °C) mentioned in the excerpt from the introduction of the paper under discussion, low temperature desalination plants do operate and have done so for a long time: The process is called MED, "Multieffect Distillation" which can, and sometimes do run on waste heat. Some have operated in places like the US Virgin Islands since before the close of the 20th century. (I'm a big fan of heat networks.) However these are not ZLD, zero liquid discharge plants. They discharge concentrated brine. A working figure for the salt content of seawater, without appeal to the sophisticated standards by which TEOS-10 operates, is 35 grams of salt per liter. The brine extracted in the Boo paper cited immediately above, contained 292 g/l, 8 times as salty as seawater. Brine this concentrated is an environmental hazard.
This differentiates the solvent extraction process from MED processes.
The paper cited at the outset of this boring post is a computer modeling paper:
Figure 1:
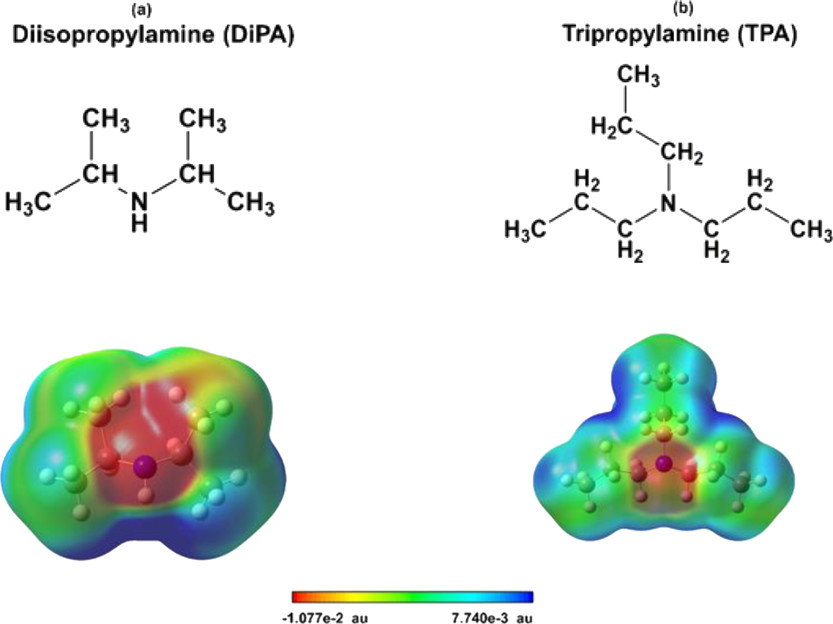
The caption:
...Two different system configurations are modeled in our investigation: (a) bulk phase solvents, used to calculate solvation free energy, and (b) binary systems (solvent–brine), used to quantify interfacial behavior. In order to create the binary systems, the solvent molecules (DiPA and TPA) are first placed in a rectangular box of 2.5 × 5 × 5 nm3 with periodic boundary conditions, followed by an equilibration stage. Then, these pre-equilibrated systems are combined to build the interfacial simulations. The biphasic system is built by placing the equilibrated organic solvent next to an equilibrated brine phase (with various salt concentrations) within a simulation box of 5 × 5 × 5 nm3. A vacuum slab is added in the z-direction. The vacuum in the system provides a single solvent–brine interface. This is to avoid the presence of two solvent interfaces interacting with the brine phase, which might elevate the water extraction. Because the temperatures explored in the simulation are well below the normal boiling points of the solvents and water, the species density in the vacuum region remains very low...
...and so on...
OK then, let's just look at the pictures:

The caption:
The caption:

The caption:
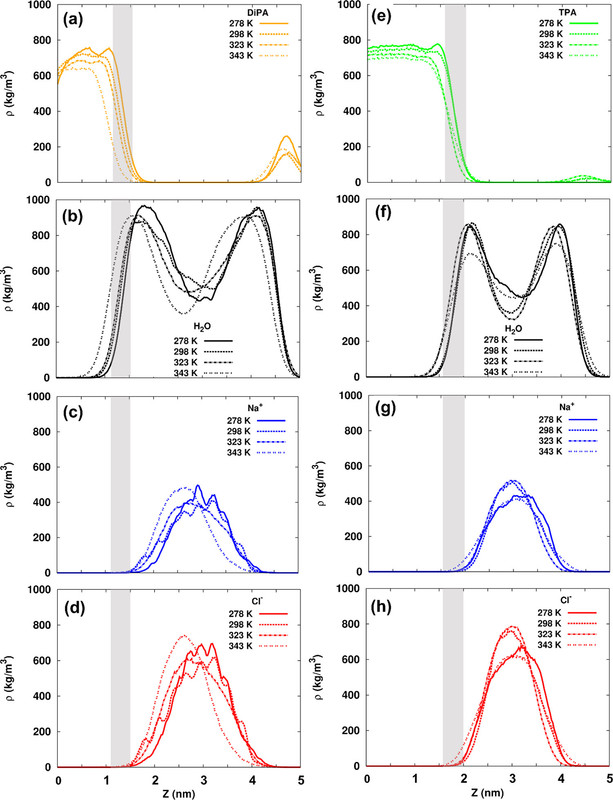
The caption:
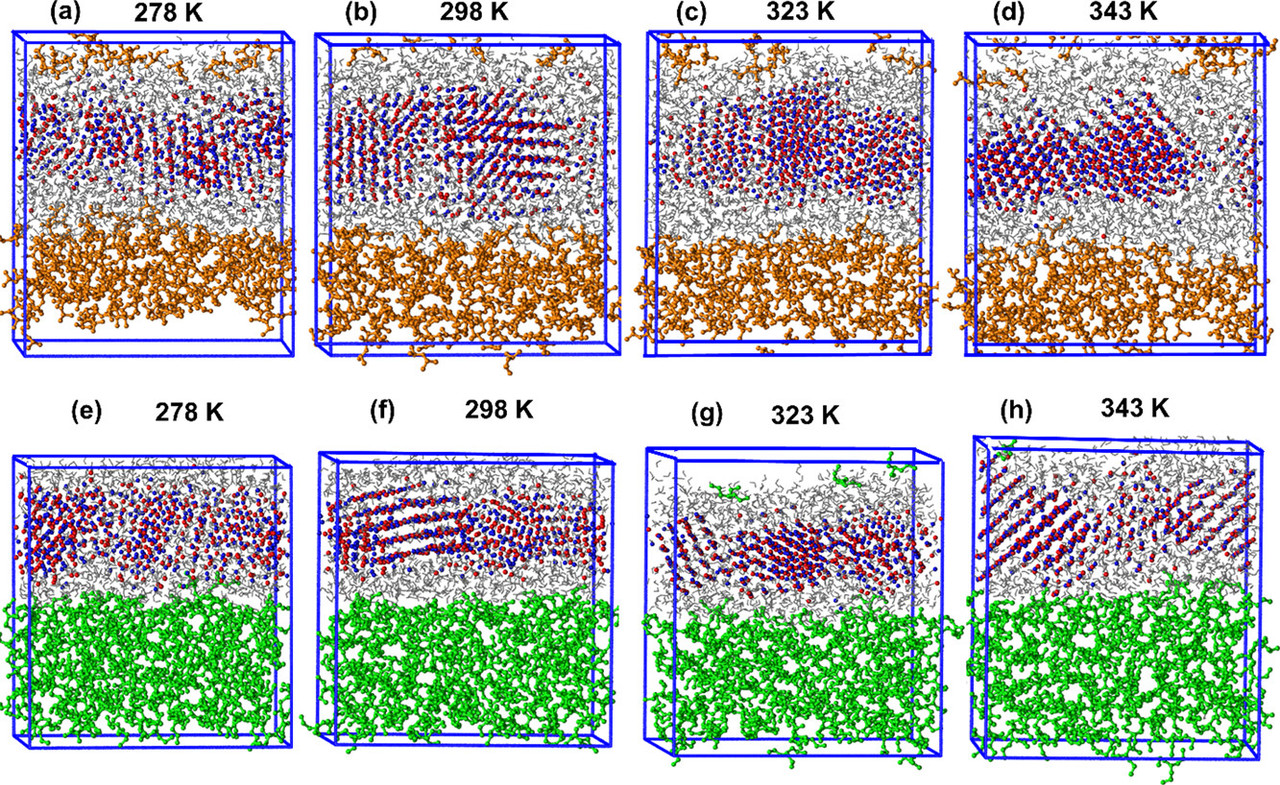
The caption:
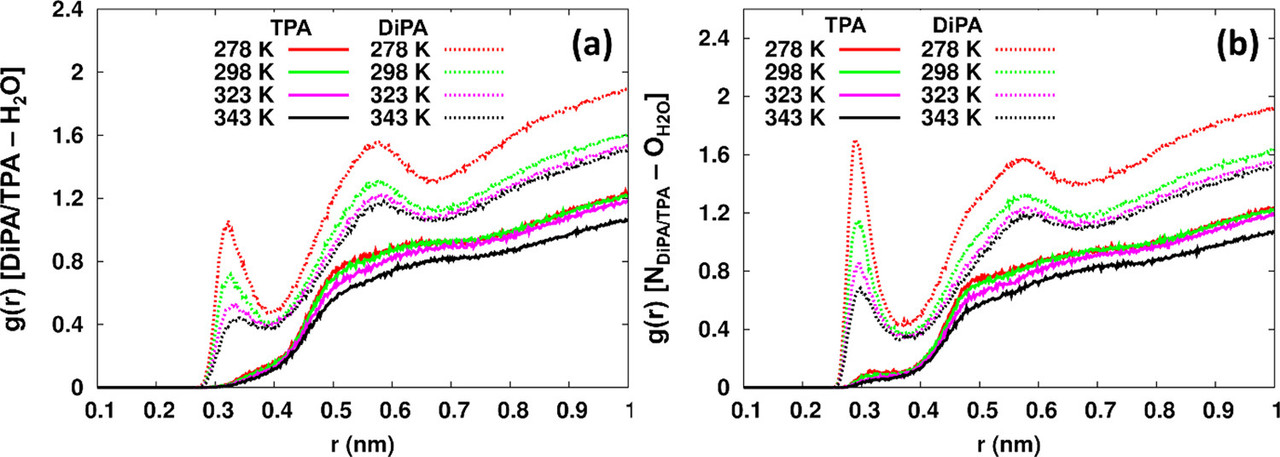
The caption:
RDF = "radial distribution function."
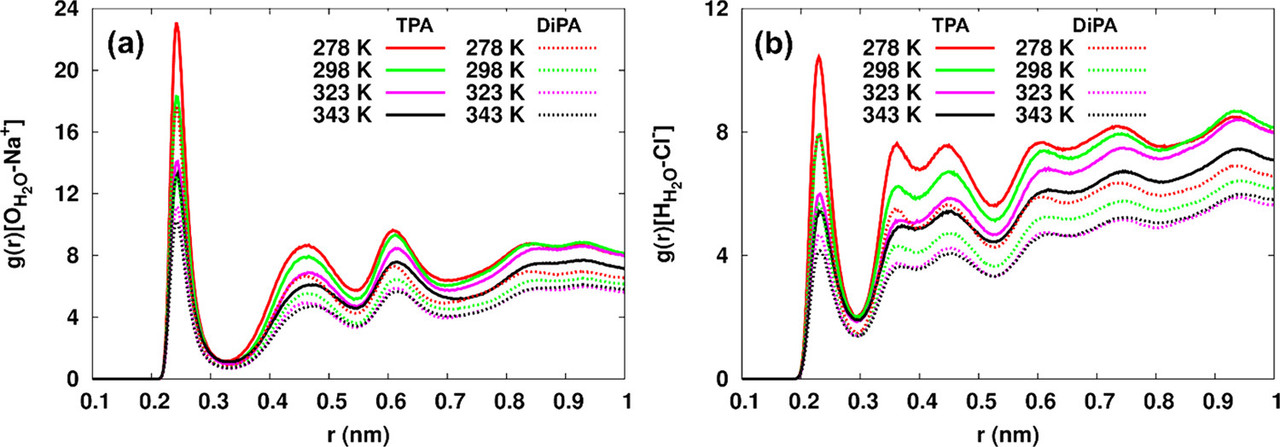
The caption:
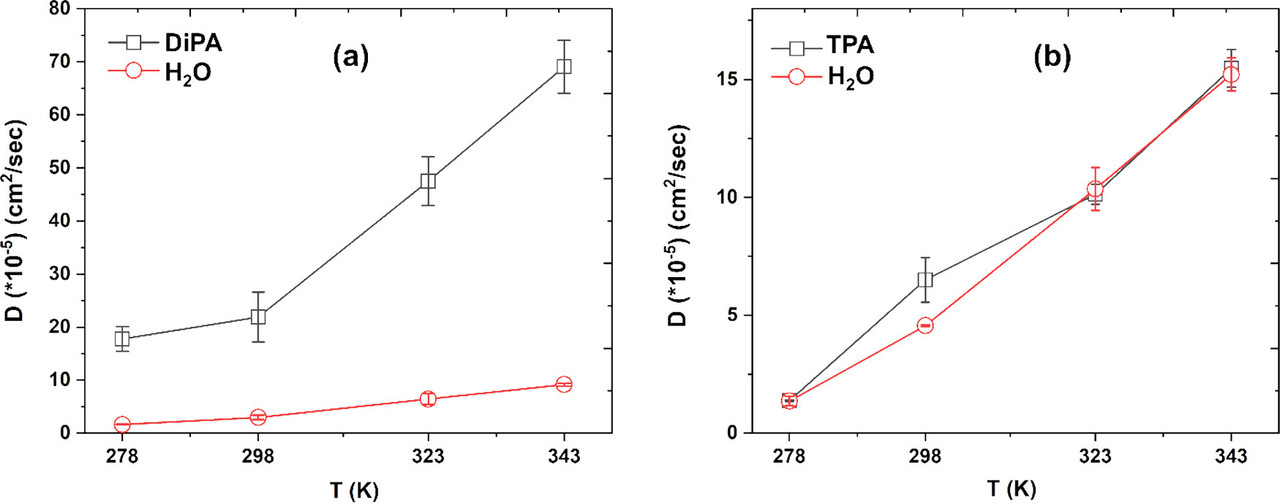
The caption:

The caption:
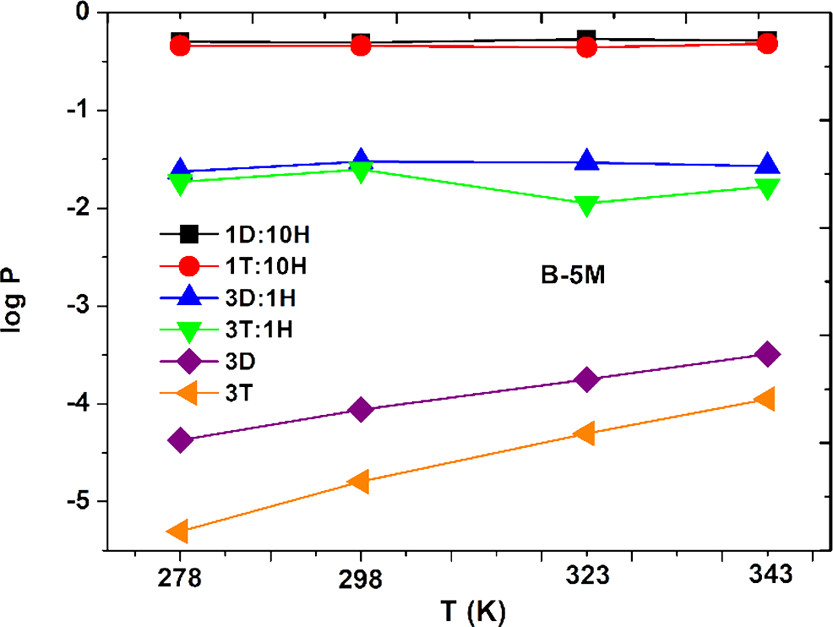
The caption:
From the conclusion:
To answer the question of "why don't we just..."
Well, there's a lot between here and there. Alkylamines smell bad, a fishy smell. Removal of residual amines from water obtained would almost certainly involve passing air through them. Our air is dirty because too many of us engage the very stupid rationale that "nuclear energy is too dangerous," and among the constituents of air pollution are the nitrogen oxides NO and NO2. Reacting with secondary amines like DPA will generate nitrosamines, many of which are potent carcinogens - dipropylnitroamine is already classified as a "suspect carcinogen." The synthetic route to alkylamines currently involves the use of dangerous fossil fuels, and the overwhelming majority of heat on this planet is generated by the combustion of dangerous fossil fuels.
If we were actually serious about phasing out dangerous fossil fuels - and very clearly we're not - we'd need to retrofit plants that we plan to close with extraction devices and heat exchangers.
In a rational world where we "went nuclear" quite literally to fight climate change - in my view the only technology with even a slim shot at addressing climate change - very high temperature nuclear reactors would be amenable to supercritical water desalination, with the added advantage of destroying microplastics in seawater, and extracting carbon dioxide from seawater. (However an SED process would make these desalination routes "ZLD" and prevent the discharge of concentrated brines into seawater.)
It is worth noting that TPA/DPA systems are certainly not the only option for SED processes featuring ZLD, something the authors note at the end of their conclusion:
These models can guide the separation of water from high-salinity brines using TSSE. Further simulations and experiments are currently underway to more broadly explore the design of task-specific amine- and imidazole-based solvents for brine treatment.
There are many good reasons for the use of imidazole based solvents, but that's a topic in an entirely different area.
This esoteric paper was a bit of a mind expander for me, and I enjoyed reading it and writing about it.
I trust your weekend will be as pleasant as mine has been thus far.